Breast Cancer Survivorship of Patients after Receiving Anti-Hormonal Therapy in Khon Kaen Hospital
Keywords:
Oral Anti-Hormonal Drugs, Survivorship, Breast CancerAbstract
This retrospective descriptive research aimed to study the survivorship of breast cancer patients after receiving oral antiretroviral therapy. The sample consisted of 620 patients with breast cancer receiving oral antiretroviral therapy who registered during the period between January 1, 2005, to December 31, 2016, and had been followed up until February 28, 2018, at Khon Kaen center of excellence for cancer. Data were collected using a patient form. Data were analyzed using frequency, percentage, standard deviation and the Kaplan – Meier method in the analysis of survival rates. Log-rank test was used in statistical tests.
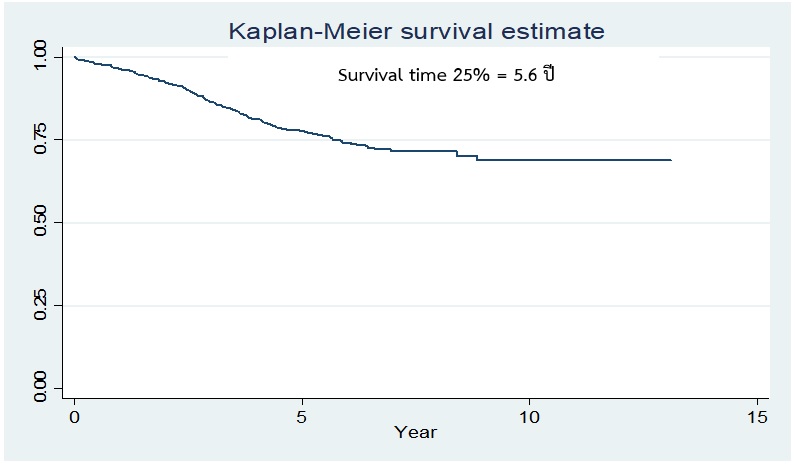
Results showed that the average age was 53.4 (SD=10.8) years, 41.90 percent received Tamoxifen only, 22.5 percent received Letozole alone and 35.55 percent had Tamoxifen together with Letozole, with the total survival rates at periods 1, 3 and 5 years equal to 96, 86 and 78 percent, respectively. The overall survival period after receiving antiretroviral therapy was 5.6 years. The two groups receiving the drugs together (Tamoxifen with Letozole) had the highest survival rate of 4.6 years. The overall survival rate at 5 years was equal to 74.7 percent (95% CI: = 68.03 to 80.19), followed by the group receiving the same type of drug (Letozole, 3.2 years). The overall 5-year survival rate was 62.9 percent (95% CI:=53.55 to 70.94). When comparing the differences of survival in each factor, it was found that the breast cancer stage 3 had a survival rate of 3.5 years and a survival rate of 5 years was 65.8 percent (95% CI: = 57.71 to 72.63), which was higher than the stage 4 group.
From the results, the study can be applied to find a guideline for survivorship care plan that increases the chances of survivorship of breast cancer patients who received anti-hormone drugs.
References
American Cancer Society. (2013). Breast Cancer Survival Rate by Stage [Online] 2013 [cited 2013 Oct 10]. Available from: http://www.cancer.org/cancer/breastcancer/ detailedguide / breast -cancer-survival-by-stage
Department of health. (1998) Reports on health and social subjects No.48. Nutritional aspects of the development of cancer. The stationery office: Norwich.
Gray, R. G., Rea D. W., Handley, K., Marshall, A., Pritchard, M. G., Perry, et al. (2008). aTTom (Adjuvant Tamoxifen-To Offermore?): Randomized Trial of 10 Versus5 Years of Adjuvant Tamoxifen among 6934 Women with Estrogen Receptor-Positive (ER+) or ER Unteste Breastcancer-Preliminary Results [abstract]. Journal Clinical Oncology, 2008; 26.
Imsamran, W. Chaiwerawattana, A., Wiangnon, S., Pongnikorn, D., Suwanrungrung, K., Sangrajrang, S., et al (2015). “Cancer in Thailand Vol VI, 2010-2012”. Bangkok: New Thammada Press(Thailand) Co., Ltd, 2015 pp 8-50.
International Agency for Research on Cancer. (2013). Globocan 2012. Retrieved Aug 7, 2013 from: http://globocan.iarc.fr/factsheet.asp
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. (2010). American Society of Clinical Oncology/ College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J ClinOncol ;28:2784- 95.
Kelsey J. L., Gammon M.D., John E.M., (1993) Reproductive fators and breas cancer. Epidemiol Rev, 15(1) 36-47.
Khuhaprema T, Attasara, P., Sriplung, H., Wiangnon, S., Sumitsawan, Y., Sangrajrang, S., (2012). Cancer in Thailand VI 2004-2006. Bangkok: National Cancer Institute; 2012.
Kongsiang, A., Tangworaphongchai, W., Chiraphonkun, C., (2014). Survival Time and Molecular Subtypes of Breast Cancer After Radiotherapy in Thailand. Asian Pacific Journal of Cancer Prevention, 15(23) 10505-1508.
Peto, R., & Davies, C. (2007). ATLAS (Adjuvant Tamoxifen, Longer Against Shorter): International Randomized Trial of 10 Versus 5 Years of Adjuvant Tamoxifen among 11, 500 Women-Preliminary Results. Presen Tation at the 30th Annual San Antonio Breast Cancer Symposium, December 13-16, 2007;
Phaluksuk, W., Srikun, P., Raksilp, M., Chueasatuchon, S., Suwanpaeng, S., Suwanrungrung, K., et al. (2014). “Cancer Registry KhonKaen Hospital-Based Report KhonKaen Hospital 2012 - 2014”. Khon Kaen: Khon kaen karn pim.
Poum, A., Kamsa-ard, S., Promthet, S. (2012). Survival Rates of Breast Cancer: A Hospital-Based Study from Northeast of Thailand. Asian Pac J Cancer Prev, 13(3), 791-4.
Supaporn, S., Kranjanalab, S., Wongkiat, S., (2004). Breast cancer. Bangkok, Phramongkutklao Hospital.
The Breast International Group. (2005). (BIG) 1-98 Collaborative Group. A Comparison of Letrozole and Tamoxifen in Postmenopausal Women with Early Breast Cancer. 2005 Dec 29;353(26):2747-57.N Engl J Med, [Online] 2017 [cited 2017 Oct 10] Available from: https://www.ncbi.nlm.nih.gov/pubmed/16382061
Downloads
Published
Issue
Section
License
1. บทความหรือข้อคิดเห็นใด ๆ ที่ปรากฏในวารสารเครือข่าย วิทยาลัยพยาบาลและการสาธารณสุขภาคใต้ ที่เป็นวรรณกรรมของผู้เขียน บรรณาธิการหรือเครือข่ายวิทยาลัยพยาบาลและวิทยาลัยการสาธารณสุขภาคใต้ ไม่จำเป็นต้องเห็นด้วย
2. บทความที่ได้รับการตีพิมพ์ถือเป็นลิขสิทธิ์ของ วารสารเครือข่ายวิทยาลัยพยาบาลและการสาธารณสุขภาคใต้







