Optimization a Guideline for Consumer Protection Using TaWai for Health Application: A Case Study of Warin Chamrap, Ubon Ratchathani
Keywords:
TaWai for Health, Consumer Protection, Management Guideline, Healthcare ProductAbstract
The objectives of this research and development aimed to study the implementation of the TaWai for Health application, a guideline for consumer protection, and to increase its efficiency using two steps of operation. The first step was to analyze the report situation from the database by using data evaluation form and management situation by interviewing 2 TaWai system administrators. The result was presented in percentage and content analysis. The second step was to increase the efficacy of TaWai for Health applications for consumer protection. The data from in-depth interviews from 6 experts and opinion hearing from 33 stakeholders were used to try out and develop the customer protection procedure using TaWai for Health application by the customer protection team in Warin Chamrap, Ubon Ratchathani, from 1 October 2018 to 31 January 2019.
The result from TaWai for Health database showed a total of 2,266 consumer protection problem reports in 3 topics, including 1,366 illegal or harmful product reports, 611 adverse product reaction reports, and 289 illegal advertisement reports. Among these reports, only 636 reports were recorded as complete reports, which accounted for 28.06 % of the total. Data analysis revealed that the low complete report rate due to irregular check and update the reports of case managers, lack of understanding of case management system, and vagueness of concrete management of daily reports. TaWai for Health networks needs the self-learning media for more understanding the application management and implementation.
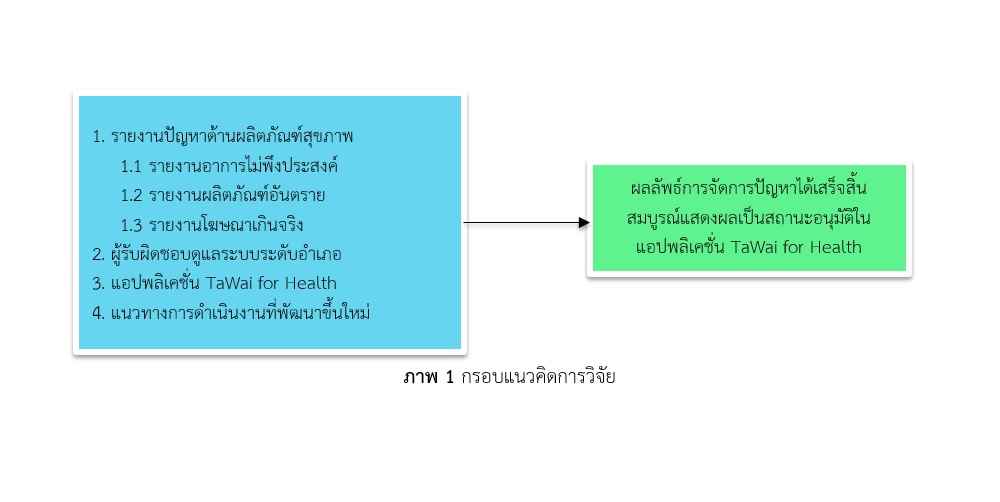
To develop the efficacy of the TaWai for Health application, the researchers created the practical management guideline applied from Surveillance and Rapid Response Time (SRRT) method. The experts and stakeholders proved the guideline before implementation. After four times of implementation and evaluation by TaWai for Health network in the study area, this procedure was practical and able to resolve the problem in time. The management guideline was including five steps. The first step was the problem report. The second step was to check and prioritize the report. The third step was problem management in order to the urgency of the problems. The fourth step is the case summary and updates of report status. The final step was the monitoring and evaluation. For an effective practice, the researcher recommends this procedure to the board of quality of life development as a practical guideline for systematic documentation and continuously controls the healthcare product problems.
References
Bureau of Epidemiology, Department of Disease Control. (2012 A). Standards and Guidelines of Surveillance and Rapid Response Team. Bangkok: The Agricultural Cooperative Federation of Thailand. Limited. (In Thai)
Bureau of Epidemiology, Department of Disease Control. (2012 B). Event Surveillance Guidelines of SRRT for Sub-District Networks. Bangkok: The Agricultural Cooperative Federation of Thailand. Limited. (In Thai)
Cosmetic Control Group, Food and Drug Administration. (2019). Dangerous Cosmetics. Retrieved May 29, 2019 from http://www.fda.moph.go.th/sites/Cosmetic/ SitePages/Dangerous_Cosmetics.aspx
Department of Medical Sciences. (2019). Alarm System Database for Quality and Safety of Healthcare Products. Retrieved May 29, 2019 from http://alert.dmsc.moph.go.th/ drug_alert_show_guest.php
Food and Drug Administration. (2016). Management Guideline for Adverse Health Care Product Problems for Public Health Officer. Retrieved May 25, 2019 from http://thaihpvc.fda.moph.go.th/thaihvc/Public/NewsAdr/uploads/hpvc_747.pdf
Kongwong, R. (2013). Screening of Non-Indication Steroidal Drug Use in Non-Communicable Disease Patients Projects in Warinchamrab, Ubon Ratchathani. (In Thai)
Laihakhot, P. (2016). Development of Surveillance Prevention and Control of Dengue Fever in the Village by SRRT District Level Ban Nong Ya Plong Tambon Bo Yai Borabue Mahasarakham. Mahasarakham Hospital Journal, 13(3), 43-50.
Mengchuay, W., & Sutheravut, P. (2010). Development Study of Customer Protection Procedures in Subdistrict Administration Organization: a Case Study of Pak Phun, Nakhon Si Thammarat. Retrieved May 25, 2019 from https://www.consumersouth.org/paper/727
Rajatanavin, R., Thakkinstian, A., Chailuekkit, L., Savanga-riyasakul, A., Sooksriwong, C., Silakawut, P., et al. (2007). Prevalence of Overt Manifestation of Steroid Abuse Without Medical Indication. Bangkok: Thailand Research Fund. (In Thai)
Downloads
Published
Issue
Section
License
1. บทความหรือข้อคิดเห็นใด ๆ ที่ปรากฏในวารสารเครือข่าย วิทยาลัยพยาบาลและการสาธารณสุขภาคใต้ ที่เป็นวรรณกรรมของผู้เขียน บรรณาธิการหรือเครือข่ายวิทยาลัยพยาบาลและวิทยาลัยการสาธารณสุขภาคใต้ ไม่จำเป็นต้องเห็นด้วย
2. บทความที่ได้รับการตีพิมพ์ถือเป็นลิขสิทธิ์ของ วารสารเครือข่ายวิทยาลัยพยาบาลและการสาธารณสุขภาคใต้







