การประเมินประสิทธิภาพการตรวจวิเคราะห์ทางโลหิตวิทยาด้วยการใช้ระบบซิกมาเมทริกซ์ กลุ่มงานเทคนิคการแพทย์ โรงพยาบาลจิตเวชขอนแก่นราชนครินทร์
คำสำคัญ:
การควบคุมคุณภาพ การประเมินความสามารถของห้องปฏิบัติการ Six Sigmaบทคัดย่อ
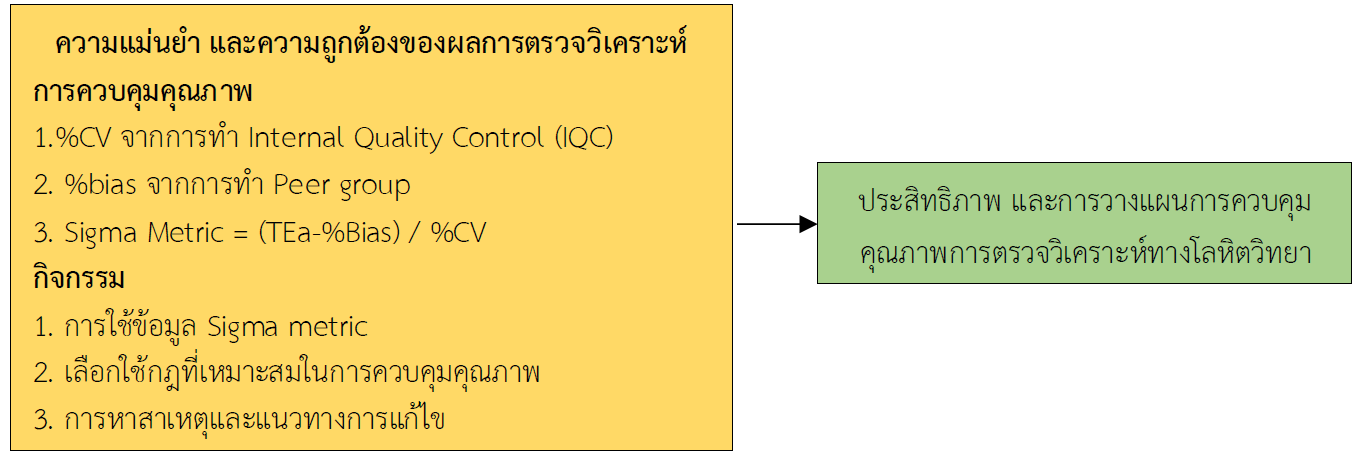
การศึกษาแบบย้อนหลังนี้เพื่อประเมินประสิทธิภาพการตรวจวิเคราะห์และวางแผนการควบคุมคุณภาพ การตรวจวิเคราะห์ทางโลหิตวิทยาโดยใช้ Sigma metric ในห้องปฏิบัติการเทคนิคการแพทย์ โรงพยาบาลจิตเวชขอนแก่นราชนครินทร์ โดยใช้ข้อมูลจากการควบคุมคุณภาพภายใน (Internal Quality Control: IQC) และการควบคุมคุณภาพภายนอก (Peer Group) ของการตรวจวิเคราะห์ทางโลหิตวิทยาคลินิกจำนวน 5 พารามิเตอร์คือ White Blood Cell (WBC), Red Blood Cell (RBC), Hemoglobin (HGB), Mean Corpuscular Volume (MCV) และ Platelet (PLT) ข้อมูลที่ใช้ในการศึกษาเก็บรวบรวมจากช่วงเวลาตั้งแต่เดือนมกราคม 2566 ถึง เดือนธันวาคม 2566 ผลการวิจัยพบว่า
มีความแม่นยำและมีความถูกต้องในการตรวจวัดอยู่ในช่วงที่ยอมรับได้ เนื่องจากค่าสัมประสิทธิ์ของความแปรปรวน (%CV) ไม่เกิน 0.33 ของค่า Total Error Allowable (TEa) ร้อยละ 100 และมีค่า %bias ไม่เกิน 0.33 ของค่า TEa ร้อยละ 100 การประเมินความสามารถของเครื่องตรวจวิเคราะห์อัตโนมัติ Beckman Coulter รุ่น Unicel DxH 800 มีค่า Sigma Metric ที่ต่ำสุดของพารามิเตอร์ HGB, RBC, WBC, MCV และ PLT เท่ากับ 4.04, 5.44, 7.97, 9.27, 10.74 ตามลำดับ สามารถเลือกใช้กฎ single rule 13s (N = 3, R = 1) พารามิเตอร์ WBC, MCV และ PLT พารามิเตอร์ RBC สามารถเลือกใช้กฎ 13s /2 of 32s/R4s (N = 3, R = 1) และพารามิเตอร์ HGB สามารถเลือกใช้กฎ 13s /2 of 32s/R4s /31s (N=3, R=2) ในการควบคุมคุณภาพ
จะเห็นได้ว่าการตรวจวิเคราะห์ที่มีความแม่นยำ และถูกต้อง ทำให้สามารถเลือกใช้กฎในการควบคุมคุณภาพที่เหมาะสมกับรายการทดสอบได้ โดยใช้กฎที่ง่ายและไม่ซับซ้อน ซึ่งช่วยประหยัดค่าใช้จ่ายจากการใช้สารควบคุมคุณภาพอีกทั้งยังลดภาระงานในการทดสอบซ้ำ และง่ายต่อการปฏิบัติงานของบุคลากร ห้องปฏิบัติการต้องติดตาม ประเมินผล และพัฒนาเทคนิคต่าง ๆ ในการควบคุมคุณภาพอย่างต่อเนื่อง
เอกสารอ้างอิง
Berta, D. M., Melku, M., Adane, T., Girma, M., Mulatie, Z., Chane, E., & Birke Teketelew, B. (2023). Analytical performance evaluation of hematology analyzer using various TEa sources and sigma metrics. Pathology and Laboratory Medicine International, 15, 65–75. doi.org/10.2147/PLMI.S414693
Cakmak, O. (2018). Evaluation of analytical performance specifications of routine clinical biochemistry tests with biological variation-based total allowable error (Tea) criteria. International Journal of Medical Biochemistry. International Journal of Medical Biochemistry, 1(3), 2-18. doi.org/10.14744/ijmb.2018.39974
El-Neanaey, A., AbdEllatif, N. M., & Abo Elwafa, R. H. (2021). Evaluation of sigma metric approach for monitoring the performance of automated analyzers in Hematology Unit of Alexandria Main University Hospital. International Journal of Laboratory Hematology, 43(6), 1388–1393. doi.org/10.1111/ijlh.13660
Katar, M., Deveci, K., & Ozmen, Z. C. (2021). Evaluation of the total quality performance of our clinical laboratory with six-sigma method. Journal of Contemporary Medicine, 11(1), 34-40. doi.org/10.16899/jcm.770304
Koutras, M. V., & Triantafyllou, I. S. (2020). Recent advances on univariate distribution-free Shewhart-type control charts. Distribution-free methods for statistical process monitoring and control, Springer International Publishing 1-56. doi.org/10.1007/978-3-030-25081-2_1
Li, M., Li, X., Lu, X., Zhong, M., Wang, L., Song, M., & Xue, F. (2023). Sigma metric used to evaluate the performance of haematology analysers: choosing an internal reference analyser for the laboratory. Hematology, 28(1), 2-3. doi.org/10.1080/16078454.2023.2277498
Llopis, M. A., Trujillo, G., Llovet, M. I., Tares, E., Ibarz, M., Bionca, C., et al. (2011). Quality indicators and specifications for key analytical-extrametrical processes in the clinical laboratory. Five years’ experience using the Six Sigma concept. Clinical Chemistry and Laboratory Medicine, 9(3), 463-470. doi.org/10.1515/cclm.2011.067
Sirithansakul, T. (2018) Assessment of hematology and clinical chemistry laboratory performance by six sigma metric; department of medical technology, Trang hospital. Technical Journal Medicine, 46(1), 6349–6374. (in Thai)
Velioglu, Y. & Yuksel, A. (2019). Complete blood count parameters in peripheral arterial disease. The Aging Male, 22(3), 187–191. doi.org/10.1080/13685538.2019.1588873
Westgard, J. O. (2017). Six sigma quality management system and design of risk-based statistical quality control. Clinics in Laboratory Medicine, 37(1), 85-96. doi.org/10.1016/j.cll.2016.09.008
ดาวน์โหลด
เผยแพร่แล้ว
ฉบับ
ประเภทบทความ
สัญญาอนุญาต
ลิขสิทธิ์ (c) 2024 วารสารเครือข่ายวิทยาลัยพยาบาลและการสาธารณสุขภาคใต้

อนุญาตภายใต้เงื่อนไข Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
1. บทความหรือข้อคิดเห็นใด ๆ ที่ปรากฏในวารสารเครือข่าย วิทยาลัยพยาบาลและการสาธารณสุขภาคใต้ ที่เป็นวรรณกรรมของผู้เขียน บรรณาธิการหรือเครือข่ายวิทยาลัยพยาบาลและวิทยาลัยการสาธารณสุขภาคใต้ ไม่จำเป็นต้องเห็นด้วย
2. บทความที่ได้รับการตีพิมพ์ถือเป็นลิขสิทธิ์ของ วารสารเครือข่ายวิทยาลัยพยาบาลและการสาธารณสุขภาคใต้







