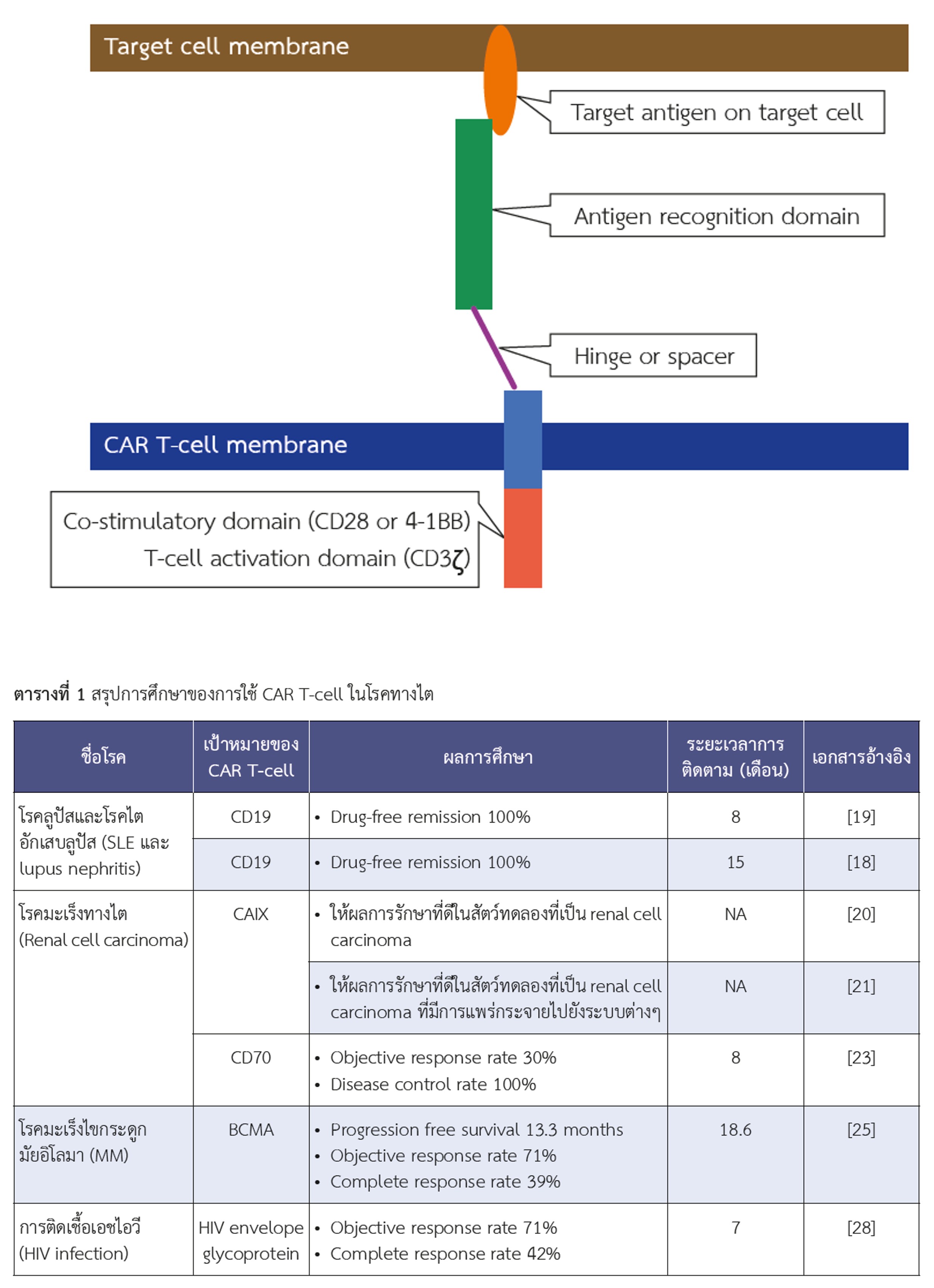
Chimeric Antigen Receptor T-cells in Kidney Disease
Main Article Content
Abstract
Chimeric antigen receptor (CAR) T-cells are T-lymphocytes genetically modified to recognize and kill specific antigens on cells, especially cancer cells. This emerging form of immunotherapy is gaining increasing significance in modern medicine. Initially developed to treat hematologic malignancies, CAR T-cell technology has evolved to target a broader range of cancers, including non-hematologic malignancies, as well as kidney-related diseases such as lupus nephritis, HIV-related kidney disease, and renal cell carcinoma. Research on CAR T-cell therapy for these conditions is growing, with more studies involving human subjects. However, as a relatively new therapeutic approach, most available data currently comes from case reports or series. Despite promising outcomes, CAR T-cell therapy can lead to severe complications, underscoring the need for large-scale studies and randomized controlled trials to establish sufficient evidence for its use in patient care.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This article is published under CC BY-NC-ND 4.0 license, which allows for non-commercial reuse of the published paper as long as the published paper is fully attributed. Anyone can share (copy and redistribute) the material in any medium or format without having to ask permission from the author or the Nephrology Society of Thailand.
References
Dabas P, Danda A. Revolutionizing cancer treatment: a comprehensive review of CAR-T cell therapy. Med Oncol 2023;40(9):275. doi: 10.1007/s12032-023-02146-y.
Zhang X, Zhu L, Zhang H, Chen S, Xiao Y. CAR-T Cell Therapy in Hematological Malignancies: Current Opportunities and Challenges. Front Immunol 2022;13:927153. doi: 10.3389/fimmu.2022.927153.
Huang J, Huang X, Huang J. CAR-T cell therapy for hematological malignancies: Limitations and optimization strategies. Front Immunol 2022;13:1019115. doi: 10.3389/fimmu.2022.1019115.
Blache U, Tretbar S, Koehl U, Mougiakakos D, Fricke S. CAR T cells for treating autoimmune diseases. RMD Open 2023;9(4). doi: 10.1136/rmdopen-2022-002907.
Wu L, Feng Y, Huang Y, Feng J, Hu Y, Huang H. CAR-T Cell Therapy: Advances in Kidney-Related Diseases. Kidney Dis (Basel) 2024;10(2):143–52. doi: 10.1159/000536194.
Chung JB, Brudno JN, Borie D, Kochenderfer JN. Chimeric antigen receptor T cell therapy for autoimmune disease. Nat Rev Immunol 2024;24(11):830–45. doi: 10.1038/s41577-024-01035-3.
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 2021;11(4):69. doi: 10.1038/s41408-021-00459-7.
Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 2013;36(2):133–51. doi: 10.1097/CJI.0b013e3182829903.
Alabanza L, Pegues M, Geldres C, Shi V, Wiltzius JJW, Sievers SA, et al. Function of Novel Anti-CD19 Chimeric Antigen Receptors with Human Variable Regions Is Affected by Hinge and Transmembrane Domains. Mol Ther 2017;25(11):2452–65. doi: 10.1016/j.ymthe.2017.07.013.
Cappell KM, Kochenderfer JN. A comparison of chimeric antigen receptors containing CD28 versus 4-1BB costimulatory domains. Nat Rev Clin Oncol 2021;18(11):715–27. doi: 10.1038/s41571-021-00530-z.
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020;396(10254):839–52. doi: 10.1016/S0140-6736(20)31366-0.
Alnefaie A, Albogami S, Asiri Y, Ahmad T, Alotaibi SS, Al-Sanea MM, et al. Chimeric Antigen Receptor T-Cells: An Overview of Concepts, Applications, Limitations, and Proposed Solutions. Front Bioeng Biotechnol 2022;10:797440. doi: 10.3389/fbioe.2022.797440.
Ayala Ceja M, Khericha M, Harris CM, Puig-Saus C, Chen YY. CAR-T cell manufacturing: Major process parameters and next-generation strategies. J Exp Med 2024;221(2). doi: 10.1084/jem.20230903.
Siegel CH, Sammaritano LR. Systemic Lupus Erythematosus: A Review. JAMA 2024;331(17):1480–91. doi: 10.1001/jama.2024.2315.
Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol 2019;71(9):1400–12. doi: 10.1002/art.40930.
Wise LM, Stohl W. Belimumab and Rituximab in Systemic Lupus Erythematosus: A Tale of Two B Cell-Targeting Agents. Front Med (Lausanne) 2020;7:303. doi: 10.3389/fmed.2020.00303.
Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N Engl J Med 2020;383(12):1117–28. doi: 10.1056/NEJMoa2001180.
Muller F, Taubmann J, Bucci L, Wilhelm A, Bergmann C, Volkl S, et al. CD19 CAR T-Cell Therapy in Autoimmune Disease - A Case Series with Follow-up. N Engl J Med 2024;390(8):687–700. doi: 10.1056/NEJMoa2308917.
Mackensen A, Muller F, Mougiakakos D, Boltz S, Wilhelm A, Aigner M, et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med 2022;28(10):2124–32. doi: 10.1038/s41591-022-02017-5.
Lo AS, Xu C, Murakami A, Marasco WA. Regression of established renal cell carcinoma in nude mice using lentivirus-transduced human T cells expressing a human anti-CAIX chimeric antigen receptor. Mol Ther Oncolytics 2014;1:14003. doi: 10.1038/mto.2014.3.
Li H, Ding J, Lu M, Liu H, Miao Y, Li L, et al. CAIX-specific CAR-T Cells and Sunitinib Show Synergistic Effects Against Metastatic Renal Cancer Models. J Immunother 2020;43(1):16–28. doi: 10.1097/CJI.0000000000000301.
Mori JI, Adachi K, Sakoda Y, Sasaki T, Goto S, Matsumoto H, et al. Anti-tumor efficacy of human anti-c-met CAR-T cells against papillary renal cell carcinoma in an orthotopic model. Cancer Sci 2021;112(4):1417–28. doi: 10.1111/cas.14835.
Srour S, Kotecha R, Curti B, Chahoud J, Drakaki A, Tang L, et al. Abstract CT011: a phase 1 multicenter study (TRAVERSE) evaluating the safety and efficacy of ALLO-316 following conditioning regimen in pts with advanced or metastatic clear cell renal cell carcinoma (ccRCC). Cancer Research 2023;83(8_Supplement):CT011–CT.
Malard F, Neri P, Bahlis NJ, Terpos E, Moukalled N, Hungria VTM, et al. Multiple myeloma. Nat Rev Dis Primers 2024;10(1):45. doi: 10.1038/s41572-024-00529-7.
Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N Engl J Med 2023;388(11):1002–14. doi: 10.1056/NEJMoa2213614.
Hansen DK, Sidana S, Peres LC, Colin Leitzinger C, Shune L, Shrewsbury A, et al. Idecabtagene Vicleucel for Relapsed/Refractory Multiple Myeloma: Real-World Experience From the Myeloma CAR T Consortium. J Clin Oncol 2023;41(11):2087–97. doi: 10.1200/JCO.22.01365.
Diana NE, Naicker S. The changing landscape of HIV-associated kidney disease. Nat Rev Nephrol 2024;20(5):330–46. doi: 10.1038/s41581-023-00801-1.
Hale M, Mesojednik T, Romano Ibarra GS, Sahni J, Bernard A, Sommer K, et al. Engineering HIV-Resistant, Anti-HIV Chimeric Antigen Receptor T Cells. Mol Ther 2017;25(3):570–9. doi: 10.1016/j.ymthe.2016.12.023.
Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016;127(26):3321– 30. doi: 10.1182/blood-2016-04-703751.
Schuster SJ, Maziarz RT, Rusch ES, Li J, Signorovitch JE, Romanov VV, et al. Grading and management of cytokine release syndrome in patients treated with tisagenlecleucel in the JULIET trial. Blood Adv 2020;4(7):1432–9. doi: 10.1182/bloodadvances.2019001304.
Sterner RC, Sterner RM. Immune effector cell associated neurotoxicity syndrome in chimeric antigen receptor-T cell therapy. Front Immunol 2022;13:879608. doi: 10.3389/fimmu.2022.879608.
Malard F, Holler E, Sandmaier BM, Huang H, Mohty M. Acute graft-versus-host disease. Nat Rev Dis Primers 2023;9(1):27. doi: 10.1038/s41572-023-00438-1.