Comparison of Amino Acid Loss Between Constant Amino Acid Plus Dextrose Infusion and Sequential Dextrose Followed by Amino Acid Infusion During Hemodialysis: A Randomized Crossover Trial
Main Article Content
Abstract
Background: Increasing amino acid loss has been observed in patients receiving intradialytic parenteral nutrition (IDPN). There are two standard protocols for lipid-free formula-IDPN infusion: constant amino acid plus dextrose infusion and sequential dextrose followed by amino acid infusion. However, the difference in amino acid loss between the two infusion protocols has never been explored.
Methods: The present study is a randomized crossover trial performed on ten malnourished chronic hemodialysis patients. They were randomized to receive a constant or sequential infusion protocol. The crossover was performed one week later. Plasma and dialysate amino acid concentrations were determined before and after the hemodialysis session. The changes in blood pressure and capillary glucose concentrations during hemodialysis were also recorded.
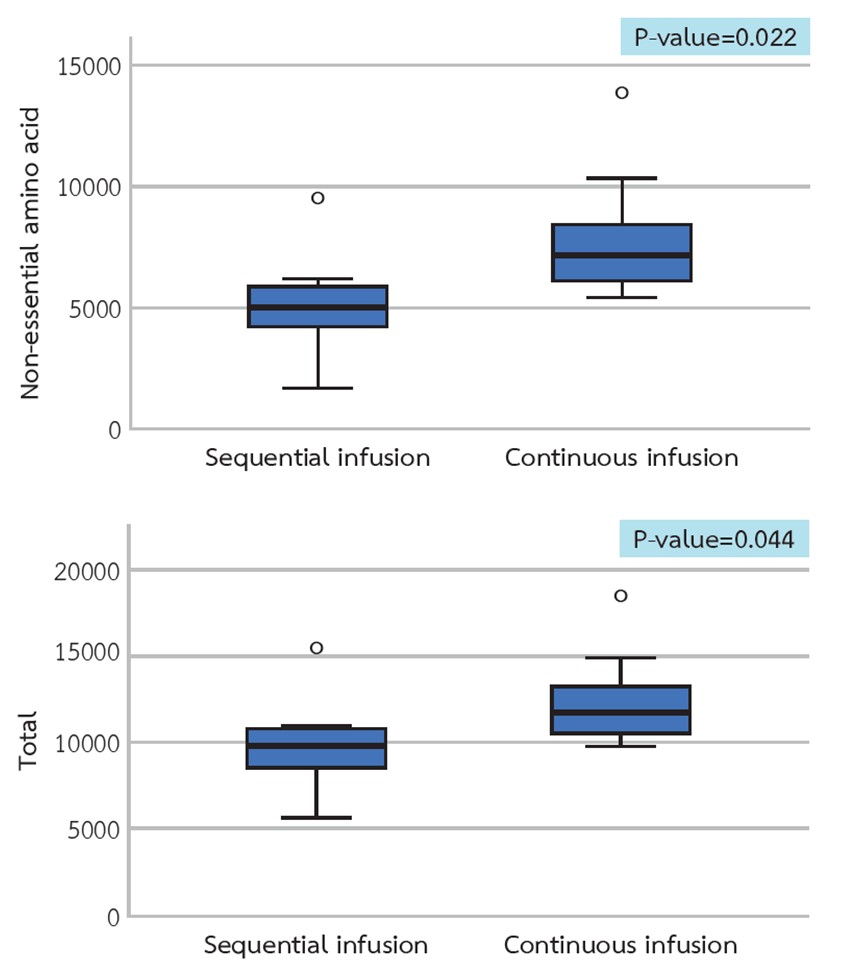
Results: The average declines in plasma essential, non-essential, and total amino acid concentrations were comparable between the two infusion protocols. Substantially higher non-essential and total amino acid concentrations were observed in the dialysate from the constant infusion group. In the sequential infusion group, the average capillary glucose level was higher at the 2nd hour and lower at the 4th hour of hemodialysis, and two patients had hypotension.
Conclusion: Constant infusion of amino acid plus dextrose solution during hemodialysis resulted in a more significant loss of amino acids into dialysate than the sequential infusion of dextrose followed by amino acids.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This article is published under CC BY-NC-ND 4.0 license, which allows for non-commercial reuse of the published paper as long as the published paper is fully attributed. Anyone can share (copy and redistribute) the material in any medium or format without having to ask permission from the author or the Nephrology Society of Thailand.
References
Hanna RM, Ghobry L, Wassef O, Rhee CM, Kalantar-Zadeh K. A Practical Approach to Nutrition, Protein-Energy Wasting, Sarcopenia, and Cachexia in Patients with Chronic Kidney Disease. Blood Purif. 2020;49(1-2):202-11.
Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr. 2013;23(2):77-90.
Lazarus JM. Nutrition in hemodialysis patients. Am J Kidney Dis. 1993;21(1):99-105.
Ikizler TA, Flakoll PJ, Parker RA, Hakim RM. Amino acid and albumin losses during hemodialysis. Kidney Int. 1994;46(3):830-7.
Navarro JF, Mora C, León C, Martín-Del Río R, Macía ML, Gallego E, et al. Amino acid losses during hemodialysis with polyacrylonitrile membranes: effect of intradialytic amino acid supplementation on plasma amino acid concentrations and nutritional variables in nondiabetic patients. Am J Clin Nutr. 2000;71(3):765-73.
Holeček M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr Metab (Lond). 2018;15:33.
Holecek M, Sprongl L, Tilser I, Tichý M. Leucine and protein metabolism in rats with chronic renal insufficiency. Exp Toxicol Pathol. 2001;53(1):71-6.
Alvestrand A, Fürst P, Bergström J. Plasma and muscle free amino acids in uremia: influence of nutrition with amino acids. Clin Nephrol. 1982;18(6):297-305.
Schauder P, Matthaei D, Henning HV, Scheler F, Langenbeck U. Blood levels of branched-chain amino acids and alpha-ketoacids in uremic patients given keto analogues of essential amino acids. Am J Clin Nutr. 1980;33(7):1660-6.
Sweatt AJ, Garcia-Espinosa MA, Wallin R, Hutson SM. Branched-chain amino acids and neurotransmitter metabolism: expression of cytosolic branched-chain aminotransferase (BCATc) in the cerebellum and hippocampus. J Comp Neurol. 2004;477(4):360-70.
Deferrari G, Garibotto G, Robaudo C, Ghiggeri GM, Tizianello A. Brain metabolism of amino acids and ammonia in patients with chronic renal insufficiency. Kidney Int. 1981;20(4):505-10.
Garibotto G, Paoletti E, Fiorini F, Russo R, Robaudo C, Deferrari G, et al. Peripheral metabolism of branched-chain keto acids in patients with chronic renal failure. Miner Electrolyte Metab. 1993;19(1):25-31.
Cano NJ. Branched-chain amino-acid metabolism in renal failure. J Ren Nutr. 2009;19(5 Suppl):S22-4.
Jensen J, Rustad PI, Kolnes AJ, Lai YC. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol. 2011;2:112.
Sacca L, Hendler R, Sherwin RS. Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab. 1978;47(5):1160-3.
Javed K, Fairweather SJ. Amino acid transporters in the regulation of insulin secretion and signalling. Biochem Soc Trans. 2019;47(2):571-90.
Wolfson M, Jones MR, Kopple JD. Amino acid losses during hemodialysis with infusion of amino acids and glucose. Kidney Int. 1982;21(3):500-6.