Efficacy and Safety of Elobixibat on Constipation in End-Stage Kidney Disease Patients Undergoing Hemodialysis: A Randomized Controlled Trial
Main Article Content
Abstract
Background: Chronic constipation is prevalent among patients with end-stage kidney disease (ESKD). Recently, elobixibat, a novel inhibitor of the ileal bile acid transporter, has been used to treat chronic constipation by stimulating bowel function. However, its efficacy and safety in ESKD patients undergoing hemodialysis (HD) have yet to be examined.
Methods: HD patients diagnosed with chronic constipation, as per the Rome IV criteria, were randomized in a 1:1 allocation ratio to receive either elobixibat or a placebo for 12 weeks. Changes in the Bristol Stool Form Scale (BSFS), frequencies of spontaneous bowel movements (SBM) and complete spontaneous bowel movements (CSBM) per week, LDL cholesterol levels, and the gut microbiota-derived metabolite, P-cresol, from baseline were evaluated after the 12-week period.
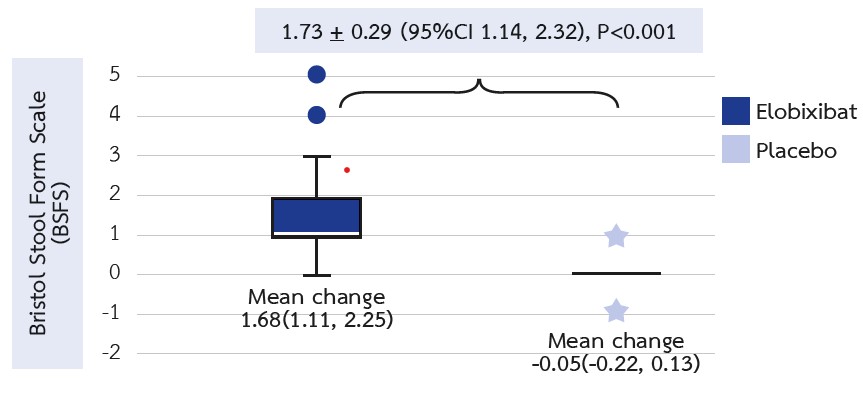
Results: A total of 46 patients participated in the study. After 12 weeks of treatment, significant improvements were observed in the BSFS (mean difference [95% confidence interval] = 1.93 [1.37, 2.48], P<0.001), and there was an increase in the frequencies of SBM (2.46 [1.69, 3.23], P<0.001) and CSBM (2.83 [1.63, 3.39], P<0.001) per week in the elobixibat group compared with the placebo group. Additionally, LDL cholesterol levels significantly decreased in the elobixibat group relative to the placebo group (-12.41 [-24.72, -0.09], P<0.001). No significant differences were noted in other laboratory data or P-cresol levels. Furthermore, no serious adverse events were reported in either group.
Conclusion: Elobixibat exhibited an efficacy in improving constipation and reducing LDL cholesterol levels. These findings suggest that elobixibat might be an effective treatment for chronic constipation in ESKD patients undergoing HD.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This article is published under CC BY-NC-ND 4.0 license, which allows for non-commercial reuse of the published paper as long as the published paper is fully attributed. Anyone can share (copy and redistribute) the material in any medium or format without having to ask permission from the author or the Nephrology Society of Thailand.
References
Masakane I, Taniguchi M, Nakai S, Tsuchida K, Wada A, Ogata S, et al. Annual Dialysis Data Report 2016, JSDT Renal Data Registry. Ren Replace Ther. 2018;4(1):45.
Strid H, Simrén M, Johansson AC, Svedlund J, Samuelsson O, Björnsson ES. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well-being. Nephrol Dial Transplant. 2002;17(8):1434-9.
Chang JY, Locke GR 3rd, McNally MA, Halder SL, Schleck CD, Zinsmeister AR, et al. Impact of functional gastrointestinal disorders on survival in the community. Am J Gastroenterol. 2010;105(4):822-32.
Honkura K, Tomata Y, Sugiyama K, Kaiho Y, Watanabe T, Zhang S, et al. Defecation frequency and cardiovascular disease mortality in Japan: The Ohsaki cohort study. Atherosclerosis. 2016;246:251-6.
Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S16-23.
Yasuda G, Shibata K, Takizawa T, Ikeda Y, Tokita Y, Umemura S, et al. Prevalence of constipation in continuous ambulatory peritoneal dialysis patients and comparison with hemodialysis patients. Am J Kidney Dis. 2002;39(6):1292-9.
Himmelfarb J. Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin Dial. 2009;22(6):636-43.
Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am J Kidney Dis. 2016;67(3):483-98.
Attaluri A, Jackson M, Valestin J, Rao SS. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol. 2010;105(6):1407-11.
Quigley EM. The enteric microbiota in the pathogenesis and management of constipation. Best Pract Res Clin Gastroenterol. 2011;25(1):119-26.
Mori H, Tack J, Suzuki H. Magnesium Oxide in Constipation. Nutrients. 2021;13(2):421.
Xing JH, Soffer EE. Adverse effects of laxatives. Dis Colon Rectum. 2001;44(8):1201-9.
Locke GR 3rd, Pemberton JH, Phillips SF. AGA technical review on constipation. American Gastroenterological Association. Gastroenterology. 2000;119(6):1766-78.
Acosta A, Camilleri M. Elobixibat and its potential role in chronic idiopathic constipation. Therap Adv Gastroenterol. 2014;7(4):167-75.
Kamei D, Kamei Y, Nagano M, Mineshima M, Nitta K, Tsuchiya K. Elobixibat alleviates chronic constipation in hemodialysis patients: a questionnaire-based study. BMC Gastroenterol. 2020;20(1):26.
Shono T, Hyakutake H. Efficacy and safety of elobixibat in hemodialysis patients with chronic constipation: a retrospective study. Ren Replace Ther. 2020;6(1):21.
Matsuyama M, Hirai K, Nonaka H, Ueda M, Morino J, Kaneko S, et al. Effects of Elobixibat on Constipation and Lipid Metabolism in Patients With Moderate to End-Stage Chronic Kidney Disease. Front Med (Lausanne). 2022;17:8:780127.
Nakajima A, Seki M, Taniguchi S, Ohta A, Gillberg PG, Mattsson JP, et al. Safety and efficacy of elobixibat for chronic constipation: results from a randomised, double-blind, placebo-controlled, phase 3 trial and an open-label, single-arm, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3(8):537-47.
Yoshinobu S, Hasuzawa N, Nagayama A, Iwata S, Yasuda J, Tokubuchi R, et al. Effects of Elobixibat, an Inhibitor of Ileal Bile Acid Transporter, on Glucose and Lipid Metabolism: A Single-arm Pilot Study in Patients with T2DM. Clin Ther. 2022;44(10):1418-26.