Potassium Binders for Hyperkalemia in Patients with Chronic Kidney Disease
Main Article Content
Abstract
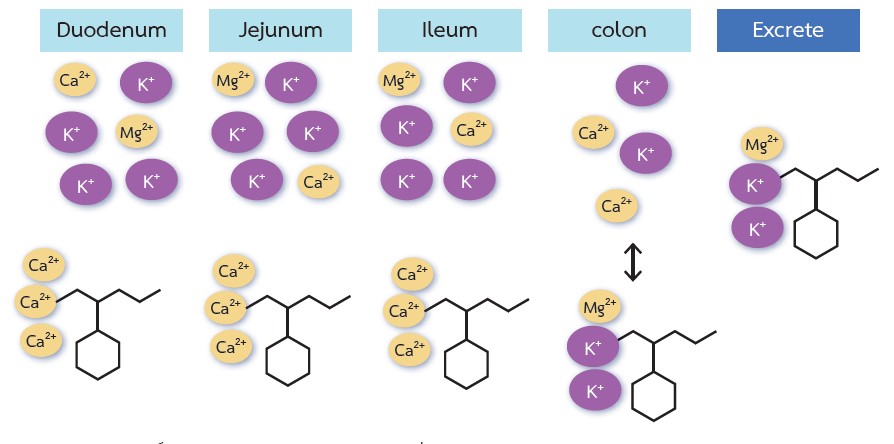
Hyperkalemia is a life-threatening complication of chronic kidney disease (CKD), particularly in patients with an estimated glomerular filtration rate of less than 45 mL/min/1.73 m2. The colon is responsible for approximately 10% of total potassium excretion, whereas the kidneys account for the remaining 90%. Nevertheless, the colon can become an important site of potassium excretion in patients with CKD. Renin-angiotensin-aldosterone system (RAAS) blockers, which can delay the progression of CKD and improve cardiovascular outcomes, usually require dose reduction or discontinuation when hyperkalemia occurs. Patiromer and sodium zirconium cyclosilicate (SZC) are potassium binders that provide alternatives to sodium polystyrene sulfonate and cause fewer gastrointestinal adverse effects. In randomized controlled studies of patients with hyperkalemia, patiromer and SZC have shown their clinical efficacy in reducing serum potassium levels with a good safety profile. These potassium binders may allow patients with CKD at risk for hyperkalemia to optimize RAAS blocker therapy. Further long-term studies are required to confirm the survival benefits of patiromer and SZC among patients with CKD.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This article is published under CC BY-NC-ND 4.0 license, which allows for non-commercial reuse of the published paper as long as the published paper is fully attributed. Anyone can share (copy and redistribute) the material in any medium or format without having to ask permission from the author or the Nephrology Society of Thailand.
References
Gilligan S, Raphael KL. Hyperkalemia and Hypokalemia in CKD: Prevalence, Risk Factors, and Clinical Outcomes. Adv Chronic Kidney Dis. 2017;24:315-8.
Luo J, Brunelli SM, Jensen DE, Yang A. Association between Serum Potassium and Outcomes in Patients with Reduced Kidney Function. Clin J Am Soc Nephrol. 2016;11:90-100.
Thomsen RW, Nicolaisen SK, Hasvold P, Sanchez RG, Pedersen L, Adelborg K, et al. Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes-a Danish population-based cohort study. Nephrol Dial Transplant. 2018;33(9):1610-20.
Núñez J, Bayés-Genís A, Zannad F, Rossignol P, Núñez E, Bodí V, et al. Long-Term Potassium Monitoring and Dynamics in Heart Failure and Risk of Mortality. Circulation. 2018;137:1320-30.
Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Sakhuja A, Mao MA, Erickson SB. Impact of admission serum potassium on mortality in patients with chronic kidney disease and cardiovascular disease. QJM. 2017;110:713-9.
Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, et al. Association of Serum Potassium with All-Cause Mortality in Patients with and without Heart Failure, Chronic Kidney Disease, and/or Diabetes. Am J Nephrol. 2017;46:213-21.
Kovesdy CP. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol. 2014;10:653-62.
Epstein M. Hyperkalemia constitutes a constraint for implementing renin-angiotensin-aldosterone inhibition: the widening gap between mandated treatment guidelines and the real-world clinical arena. Kidney Int Suppl (2011). 2016;6:20-8.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137-61.
Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019; 42 Suppl 1:S103-23.
Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21 Suppl 11:S212-20.
Palmer BF, Clegg DJ. Physiology and Pathophysiology of Potassium Homeostasis: Core Curriculum 2019. Am J Kidney Dis. 2019;74:682-95.
Tamargo J, Caballero R, Delpon E. New drugs for the treatment of hyperkalemia in patients treated with renin-angiotensinaldosterone system inhibitors--hype or hope?. Discovery Medicine. 2014 Nov 1;18(100):249-54.
Mathialahan T, Maclennan KA, Sandle LN, Verbeke C, Sandle GI. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol. 2005;206:46-51.
van Dinter TG, Jr., Fuerst FC, Richardson CT, Ana CA, Polter DE, Fordtran JS, et al. Stimulated active potassium secretion in a patient with colonic pseudo-obstruction: a new mechanism of secretory diarrhea. Gastroenterology. 2005;129:1268-73.
Yamada S, Inaba M. Potassium Metabolism and Management in Patients with CKD. Nutrients. 2021;13(6):1751.
Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126:264.e9-24.
Wong SWS, Zhang G, Norman P, Welihinda H, Wijeratne DT. Polysulfonate Resins in Hyperkalemia: A Systematic Review. Can J Kidney Health Dis. 2020;7:2054358120965838.
U.S. Food and Drug Administration. Drug Approvals and Databases:VELTASSA 2015 [December 18, 2022]. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ucm474619.htm.
Li L, Harrison SD, Cope MJ, Park C, Lee L, Salaymeh F, et al. Mechanism of Action and Pharmacology of Patiromer, a Nonabsorbed Cross-Linked Polymer That Lowers Serum Potassium Concentration in Patients With Hyperkalemia. J Cardiovasc Pharmacol Ther. 2016;21:456-65.
Lesko LJ, Offman E, Brew CT, Garza D, Benton W, Mayo MR, et al. Evaluation of the Potential for Drug Interactions With Patiromer in Healthy Volunteers. J Cardiovasc Pharmacol Ther. 2017;22(5):434-46.
Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebocontrolled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820-8.
Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211-21.
Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, et al. Effect of Patiromer on Serum Potassium Level in Patients With Hyperkalemia and Diabetic Kidney Disease: The AMETHYST-DN Randomized Clinical Trial. JAMA. 2015;314:151-61.
Kovesdy CP, Rowan CG, Conrad A, Spiegel DM, Fogli J, Oestreicher N, et al. Real-World Evaluation of Patiromer for the Treatment of Hyperkalemia in Hemodialysis Patients. Kidney Int Rep. 2019;4(2):301-9.
Butler J, Anker SD, Lund LH, Coats AJS, Filippatos G, Siddiqi TJ, et al. Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial. Eur Heart J. 2022;43(41):4362-73.
Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic Review and Meta-Analysis of Patiromer and Sodium Zirconium Cyclosilicate: A New Armamentarium for the Treatment of Hyperkalemia. Pharmacotherapy. 2017;37:401-11.
Shrestha DB, Budhathoki P, Sedhai YR, Baniya R, Cable CA, Kashiouris MG, et al. Patiromer and Sodium Zirconium Cyclosilicate in Treatment of Hyperkalemia: A Systematic Review and Meta-Analysis. Curr Ther Res Clin Exp. 2021;95:100635.
Chaitman M, Dixit D, Bridgeman MB. Potassium-Binding Agents for the Clinical Management of Hyperkalemia. P T. 2016;41:43-50.
U.S. Food and Drug Administration. Drug Approvals and Databases:LOKELMA 2018 [December 18, 2022]. Available from: https://www.fda.gov/Drugs/InformationOnDrugs/ucm609666.html.
Stavros F, Yang A, Leon A, Nuttall M, Rasmussen HS. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One. 2014;9:e114686.
Fishbane S, Ford M, Fukagawa M, McCafferty K, Rastogi A, Spinowitz B, et al. A Phase 3b, Randomized, Double-Blind, Placebo-Controlled Study of Sodium Zirconium Cyclosilicate for Reducing the Incidence of Predialysis Hyperkalemia. J Am Soc Nephrol. 2019;30:1723-33.
Ash SR, Singh B, Lavin PT, Stavros F, Rasmussen HS. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int. 2015;88:404-11.
Kosiborod M, Rasmussen HS, Lavin P, Qunibi WY, Spinowitz B, Packham D, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312:2223-33.
Roger SD, Spinowitz BS, Lerma EV, Fishbane S, Ash SR, Martins JG, et al. Sodium zirconium cyclosilicate increases serum bicarbonate concentrations among patients with hyperkalaemia: exploratory analyses from three randomized, multi-dose, placebo-controlled trials. Nephrol Dial Transplant. 2021;36:871-83.
Roger SD, Spinowitz BS, Lerma EV, Singh B, Packham DK, Al-Shurbaji A, et al. Efficacy and Safety of Sodium Zirconium Cyclosilicate for Treatment of Hyperkalemia: An 11-Month Open-Label Extension of HARMONIZE. Am J Nephrol. 2019;50:473-80.
Anker SD, Kosiborod M, Zannad F, Piña IL, McCullough PA, Filippatos G, et al. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail. 2015;17:1050-6.
Peacock WF, Rafique Z, Vishnevskiy K, Michelson E, Vishneva E, Zvereva T, et al. Emergency Potassium Normalization Treatment Including Sodium Zirconium Cyclosilicate: A Phase II, Randomized, Double-blind, Placebo-controlled Study (ENERGIZE). Acad Emerg Med. 2020;27:475-86.
Spinowitz BS, Fishbane S, Pergola PE, Roger SD, Lerma EV, Butler J, et al. Sodium Zirconium Cyclosilicate among Individuals with Hyperkalemia: A 12-Month Phase 3 Study. Clin J Am Soc Nephrol. 2019;14(6):798-809.
Roger SD, Lavin PT, Lerma EV, McCullough PA, Butler J, Spinowitz BS, et al. Long-term safety and efficacy of sodium zirconium cyclosilicate for hyperkalaemia in patients with mild/moderate versus severe/end-stage chronic kidney disease: comparative results from an open-label, Phase 3 study. Nephrol Dial Transplant. 2021;36(1):137-50.