Oxalate Nephropathy
Main Article Content
Abstract
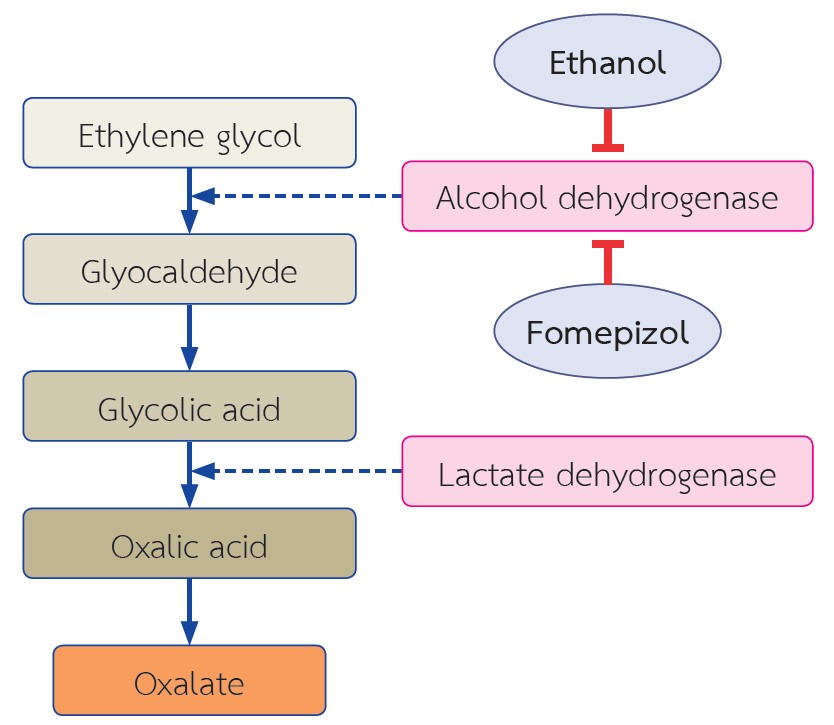
The exact function of oxalate in the body is unknown. Eighty percent of blood oxalate is synthesized by enzymes in the liver and the rest is derived from diet. Factors that influence oxalate absorption from the intestine include dietary calcium and fat intake and intestinal microflora. The main route of oxalate excretion is through the kidney. In the situation where oxalate metabolism is impaired such as hereditary disorder or disorders of the gastrointestinal tract, urinary oxalate excretion increases to compensate for the increase in circulating oxalate. The increase in oxalate concentration in renal parenchyma and tubules can result in many forms of kidney dysfunction including acute and chronic oxalate nephropathy or calcium oxalate urolithiasis. Prolonged exposure to oxalate can cause permanent kidney damage and chronic kidney disease. Further impairment in oxalate excretion leads to oxalate accumulation in other organs such as eyes, bone and heart. Kidney biopsy can reveal the presence of oxalate crystals in renal
parenchyma and tubules accompanied by tubular injury and damage. Current evidence suggest that calcium oxalate crystal may trigger interleukin-1β-dependent innate immunity via the NLRP3 in intrarenal mononuclear phagocytes and directly damage tubular cells. Specific treatments including liver and/or kidney transplantation or pancreatic enzyme supplementation can eliminate oxalate accumulation. Other supportive care include increased water intake, calcium supplement and dietary fat and oxalate restriction. Among patients who receive hemodialysis, blood oxalate should be kept at the lowest level in order to prevent oxalate accumulation in other organs.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This article is published under CC BY-NC-ND 4.0 license, which allows for non-commercial reuse of the published paper as long as the published paper is fully attributed. Anyone can share (copy and redistribute) the material in any medium or format without having to ask permission from the author or the Nephrology Society of Thailand.
References
Buysschaert B, Aydin S, Morelle J, Gillion V, Jadoul M, Demoulin N. Etiologies, Clinical Features, and Outcome of Oxalate Nephropathy. Kidney Int Rep. 2020;5:1503-9.
Demoulin N, Aydin S, Gillion V, Morelle J, Jadoul M. Pathophysiology and Management of Hyperoxaluria and Oxalate Nephropathy: A Review. American Journal of Kidney Diseases. Forthcoming 2022.
Huang Y, Zhang YH, Chi ZP, Huang R, Huang H, Liu G, et al. The handling of oxalate in the body and the origin of oxalate in calcium oxalate stones. Urologia internationalis. 2020;104:167-76.
Chanapa P. The risk factors of kidney stones focusing on calcium and oxalate. Songklanagarind Medical Journal. 2012;29:299-308.
Ferrara L. Averrhoa carambola Linn: Is it really a toxic fruit? International Journal of Medical Reviews. 2018;5:2-5.
Liu M, Nazzal L. Enteric hyperoxaluria: role of microbiota and antibiotics. Current opinion in nephrology and hypertension. 2019;28:352-9.
Dawson PA, Russell CS, Lee S, McLeay SC, van Dongen JM, Cowley DM, et al. Urolithiasis and hepatotoxicity are linked to the anion transporter Sat1 in mice. The Journal of clinical investigation. 2010;120:706-12.
Beck BB, Hoyer-Kuhn H, Göbel H, Habbig S, Hoppe B. Hyperoxaluria and systemic oxalosis: an update on current therapy and future directions. Expert opinion on investigational drugs. 2013;22:117-29.
Anders H-J, Suarez-Alvarez B, Grigorescu M, Foresto-Neto O, Steiger S, Desai J, et al. The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1–mediated tissue injury. Kidney international. 2018;93:656-69.
Glew RH, Sun Y, Horowitz BL, Konstantinov KN, Barry M, Fair JR, et al. Nephropathy in dietary hyperoxaluria: A potentially preventable acute or chronic kidney disease. World journal of nephrology. 2014;3:122.
Geraghty R, Wood K, Sayer J. Calcium oxalate crystal deposition in the kidney: identification, causes and consequences. Urolithiasis. 2020;48:1-8.
Mulay SR, Anders H-J. Crystallopathies. New England Journal of Medicine. 2016;374:2465-76.
Ermer T, Eckardt K-U, Aronson PS, Knauf F. Oxalate, inflammasome, and progression of kidney disease. Current opinion in nephrology and hypertension. 2016;25:363.
Fogo AB, Lusco MA, Najafian B, Alpers CE. AJKD atlas of renal pathology: oxalosis. American Journal of Kidney Diseases. 2017;69:e13-e4.
Bhasin B, Ürekli HM, Atta MG. Primary and secondary hyperoxaluria: Understanding the enigma. World journal of nephrology. 2015;4:235.
Milliner DS, Harris PC, Cogal AG, Lieske JC. Primary hyperoxaluria type 1. 2017;25:1-37.
Coulter-Mackie MB, White CT, Hurley RM, Chew BH, Lange D. Primary hyperoxaluria type 1. Gene ReviewsTM [Internet]. In: Pagon RA, Ardinger HH. Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. Seattle (Washington):University of Washington, 1993-2013.
Monico CG, Persson M, Ford GC, Rumsby G, Milliner DS. Potential mechanisms of marked hyperoxaluria not due to primary hyperoxaluria I or II. Kidney international. 2002;62:392-400.
Ramaswamy C, Williams J, Griffiths D. Reversible acute renal failure with calcium oxalate cast nephropathy—possible role of ascorbic acid. Nephrology Dialysis Transplantation. 1993;8:1387-9.
Parry MF, Wallach R. Ethylene glycol poisoning. The American journal of medicine. 1974;57:143-50.
Frascino JA, Vanamee P, Rosen PP. Renal oxalosis and azotemia after methoxyflurane anesthesia. New England Journal of Medicine. 1970;283:676-9.
Cochat P, Rumsby G. Primary hyperoxaluria. New England Journal of Medicine. 2013;369:649-58.
Rosenstock JL, Joab TM, DeVita MV, Yang Y, Sharma PD, Bijol V. Oxalate nephropathy: a review. Clinical Kidney Journal. 2021;14:1859-993.