Development of a simple HPLC method for the determination of urinary O-aminohippuric acid (OAH) and an establishment of OAH reference interval
Main Article Content
Abstract
Background: O-aminohippuric acid (OAH) is considered a low-abundance urinary fluorescent metabolite with the potential to be an innovative lung cancer biomarker. There is a lack of simple methods for measuring urinary OAH metabolite, and the measurement needs to be normalized by urinary creatinine, which is produced at a constant rate. Thus, the newly developed method must be able to determine urinary creatinine and OAH simultaneously in the same run.
Objective: This work aimed to develop and validate a simple high-performance liquid chromatography (HPLC) method for measuring urinary OAH and to establish the reference intervals of OAH in healthy individuals.
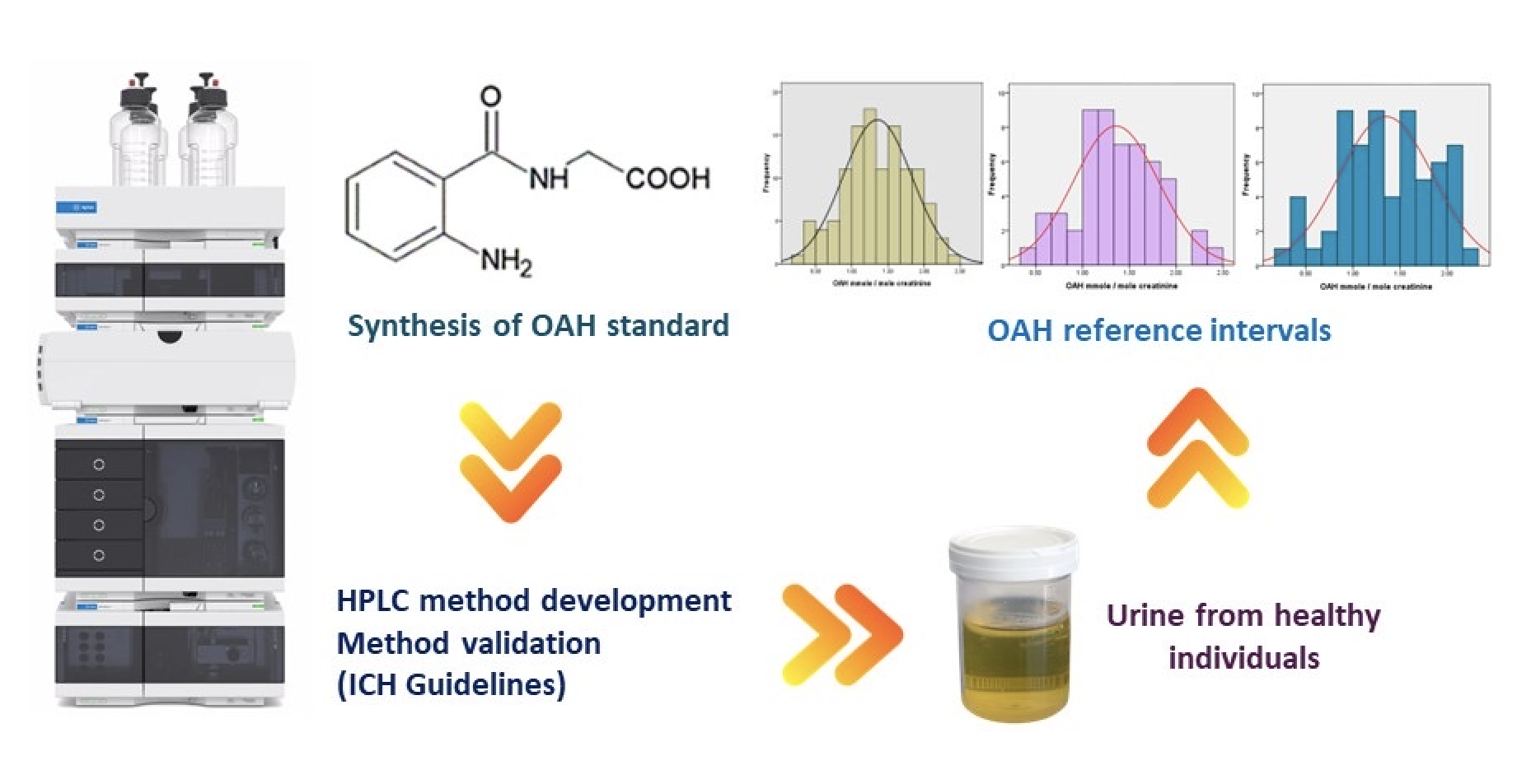
Materials and methods: We synthesized OAH standard in a simple route and optimized simple HPLC method for simultaneous measurement of creatinine and OAH in a single run. Analysis was performed on a RP-C18 column with a gradient elution system of acetonitrile - ammonium acetate buffer (pH 6.5, 100 mM). After implementing optimal conditions, the procedure was compiled according to the International Council for Harmonisation (ICH) validation parameters. The developed method was used for the establishment of reference intervals of a total of 120 random urine samples of healthy individuals.
Results: The linear range of the calibration curve for creatinine and OAH were 1-1000 µg/mL and 0.1-100 µg/mL, respectively. The recoveries ranged for both metabolites were between 91.35 % and 109.12%. The relative standard deviations (RSDs) for the intra-day and inter-day results ranged from 0.11-0.66 % to 0.16-1.73 %, respectively. The limits of detection (LOD) and quantification (LOQ) were 0.258 µg/mL and 0.783 µg/mL for creatinine, while OAH was 0.045 µg/mL and 0.137 µg/mL, respectively. The method was successfully applied to establish reference intervals of OAH in healthy individuals and was defined as 0.420-2.287 mmol/mol creatinine.
Conclusion: According to various validated parameters, the proposed method was proven to quantify urinary OAH and creatinine in a single run. It can also be analyzed noninvasively without additional sample processing. Reported herein is the first establishment of OAH reference intervals in healthy individuals, which may benefit the utilization of OAH as a noninvasive biomarker for lung cancer detection in the future.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Funai K, Honzawa K, Suzuki M, Momiki S, Asai K, Kasamatsu N, et al. Urinary fluorescent metabolite O-aminohippuric acid is a useful biomarker for lung cancer detection. Metabolomics. 2020; 16(10): 101. doi: 10.1007/s11306-020-01721-y.
An Z, Chen Y, Zhang R, Song Y, Sun J, He J, et al. Integrated ionization approach for RRLC-MS/MSbased metabonomics: finding potential biomarkers for lung cancer. J Proteome Res. 2010; 9(8): 4071-81. doi: 10.1021/pr100265g.
Wang Q, Liu D, Song P, Zou MH. Tryptophankynurenine pathway is dysregulated in inflammation, and immune activation. Front Biosci (Landmark Ed). 2015; 20(7): 1116-43. doi: 10.2741/4363.
Chuang SC, Fanidi A, Ueland PM, Relton C, Midttun O, Vollset SE, et al. Circulating biomarkers of tryptophan and the kynurenine pathway and lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2014; 23(3): 461-8. doi: 10.1158/1055-9965.Epi-13-0770.
Walczak K, Wnorowski A, Turski WA, Plech T. Kynurenic acid and cancer: facts and controversies. Cell Mol Life Sci. 2020; 77(8): 1531-50. doi: 10.1007/s00018-019-03332-w.
Mor A, Tankiewicz-Kwedlo A, Pawlak D. Kynurenines as a novel target for the treatment of malignancies. Pharmaceuticals (Basel). 2021;14(7): 606. doi: 10.3390/ph14070606.
Schmidt DR, Patel R, Kirsch DG, Lewis CA, Vander Heiden MG, Locasale JW. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J Clin. 2021; 71(4): 333-58. doi: 10.3322/caac.21670.
Armitage EG, Southam AD. Monitoring cancer prognosis, diagnosis and treatment efficacy using metabolomics and lipidomics. Metabolomics. 2016; 12(9): 146. doi: 10.1007/s11306-016-1093-7.
Kannampuzha S, Mukherjee AG, Wanjari UR, Gopalakrishnan AV, Murali R, Namachivayam A, et al. A systematic role of metabolomics, metabolic pathways, and chemical metabolism in lung cancer. Vaccines (Basel). 2023;11(2):381. doi: 10.3390/vaccines11020381.
Liesenfeld DB, Habermann N, Owen RW, Scalbert A, Ulrich CM. Review of mass spectrometry-based metabolomics in cancer research. Cancer Epidemiol Biomarkers Prev. 2013; 22(12): 2182-201. doi: 10.1158/10559965.Epi-13-0584.
Lee KB, Ang L, Yau WP, Seow WJ. Association between metabolites and the risk of lung cancer: a systematic literature review and meta-analysis of observational studies. Metabolites. 2020; 10(9): 362. doi: 10.3390/metabo10090362.
Gasparri R, Sedda G, Caminiti V, Maisonneuve P, Prisciandaro E, Spaggiari L. Urinary biomarkers for early diagnosis of lung cancer. J Clin Med. 2021; 10(8): 1723. doi: 10.3390/jcm10081723.
Jordaens S, Zwaenepoel K, Tjalma W, Deben C, Beyers K, Vankerckhoven V, et al. Urine biomarkers in cancer detection: a systematic review of preanalytical parameters and applied methods. Int J Cancer. 2023; 152(10): 2186-205. doi: 10.1002/ijc.34434.
Kalantari K, Bolton WK. A good reason to measure 24-hour urine creatinine excretion, but not to assess kidney function. Clin J Am Soc Nephrol. 2013; 8(11): 1847-9. doi: 10.2215/cjn.09770913.
Khamis MM, Adamko DJ, El-Aneed A. Mass spectrometric based approaches in urine metabolomics and biomarker discovery. Mass Spectrom Rev. 2017; 36(2): 115-34. doi: 10.1002/mas.21455.
Yang Q, Shi X, Wang Y, Wang W, He H, Lu X, et al. Urinary metabonomic study of lung cancer by a fully automatic hyphenated hydrophilic interaction/RPLCMS system. J Sep Sci. 2010; 33(10): 1495-503. doi: 10.1002/jssc.200900798.
Pearson MA, Lu C, Schmotzer BJ, Waller LA, Riederer AM. Evaluation of physiological measures for correcting variation in urinary output: Implications for assessing environmental chemical exposure in children. J Expo Sci Environ Epidemiol. 2009; 19(3): 336-42. doi: 10.1038/jes.2008.48.
Wijemanne N, Soysa P, Wijesundara S, Perera H. Development and validation of a simple high performance liquid chromatography/UV method for simultaneous determination of urinary uric acid, hypoxanthine, and creatinine in human urine. Int J Anal Chem. 2018; 2018: 1647923. doi: 10.1155/2018/1647923.
Garde AH, Hansen AM, Kristiansen J, Knudsen LE. Comparison of uncertainties related to standardization of urine samples with volume and creatinine concentration. Ann Occup Hyg. 2004; 48(2): 171-9. doi: 10.1093/annhyg/meh019.
Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993; 54(10): 615-27. doi: 10.1080/15298669391355134.
Sutariya V, Wehrung D, Geldenhuys WJ. Development and validation of a novel RP-HPLC method for the analysis of reduced glutathione. J Chromatogr Sci. 2012; 50(3): 271-6. doi: 10.1093/chromsci/bmr055.
Bijukumar G, Maloyesh B, Sampat S, Bhirud SB, Rajendra A. Efficient synthesis of sivelestat sodium hydrate. Synthetic Communications. 2008; 38: 1718-24. doi: 10.1080/00397910801982373
Locke D, Bevans CG, Wang LX, Zhang Y, Harris AL, Lee YC. Neutral, acidic, and basic derivatives of anthranilamide that confer different formal charge to reducing oligosaccharides. Carbohydr Res. 2004; 339(2): 221-31. doi: 10.1016/j.carres.2003.10.020.
Morimoto K, Nishimura K, Miyasaka S, Maeta H, Taniguchi I. The effect of sivelestat sodium hydrate on severe respiratory failure after thoracic aortic surgery with deep hypothermia. Ann Thorac Cardiovasc Surg. 2011; 17(4): 369-75. doi: 10.5761/atcs.oa.10.01555.
Resines JA, Arín MJ, Díez MT. Determination of creatinine and purine derivatives in ruminants’ urine by reversed-phase high-performance liquid chromatography. J Chromatogr. 1992; 607(2): 199- 202. doi: 10.1016/00219673(92)87075-j.
Yokoyama Y, Tsuchiya M, Sato H, Kakinuma H. Determination of creatinine and ultraviolet-absorbing amino acids and organic acids in urine by reversedphase high-performance liquid chromatography. J Chromatogr. 1992; 583(1): 1-10. doi: 10.1016/0378- 4347(92)80338-q.
Zuo Y, Wang C, Zhou J, Sachdeva A, Ruelos VC. Simultaneous determination of creatinine and uric acid in human urine by high-performance liquid chromatography. Anal Sci. 2008; 24(12): 1589-92. doi: 10.2116/analsci.24.1589.
Muthusamy S, Palanisamy S, Ramalingam S. Exposure of bisphenol A in breast cancer patients-quantitatively assessed by sensitivity-enhanced high-performance liquid chromatography coupled with fluorescence detection: A case-control study. Biomed Chromatogr. 2021; 35(9): e5137. doi: 10.1002/bmc.5137.
Pawul-Gruba M, Kiljanek T, Madejska A, Osek J. Development of a high performance liquid chromatography with diode array detector (HPLCDAD) method for determination of biogenic amines in ripened cheeses. Molecules. 2022; 27(23): 8194. doi: 10.3390/molecules27238194.
Martínez-Navarro EM, Cebrián-Tarancón C, MoratallaLópez N, Lorenzo C, Alonso GL, Salinas RM. Development and validation of an HPLC-DAD method for determination of oleuropein and other bioactive compounds in olive leaf by-products. J Sci Food Agric. 2021; 101(4): 1447-53. doi: 10.1002/jsfa.10758.
Solano-Cueva N, Figueroa JG, Loja C, Armijos C, Vidari G, Ramírez J. A Validated HPLC-UV-ESI-IT-MS method for the quantification of carnosol in lepechinia mutica, a medicinal plant endemic to ecuador. Molecules. 2023; 28(18): 6701. doi: 10.3390/molecules28186701.
Jîtcă G, Fogarasi E, Ősz BE, Vari CE, Tero-Vescan A, Miklos A, et al. A simple HPLC/DAD method validation for the quantification of malondialdehyde in rodent’s brain. Molecules. 2021; 26(16): 5066. doi: 10.3390/molecules26165066.
Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol. 2009; 20(2): 436-43. doi.org/10.1681/asn.2008030292.
Birková A, Valko-Rokytovská M, Hubková B, Zábavníková M, Mareková M. Strong dependence between tryptophan-related fluorescence of urine and malignant melanoma. Int J Mol Sci. 2021; 22(4): 1884. doi: 10.3390/ijms22041884.
Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, et al. The human urine metabolome. PLoS One. 2013; 8(9): e73076. doi: 10.1371/journal.pone.0073076.