A genetically engineered mouse/human chimeric antibody targeting CD99 enhances antibody-dependent cellular phagocytosis against human mantle cell lymphoma Z138 cells
Main Article Content
Abstract
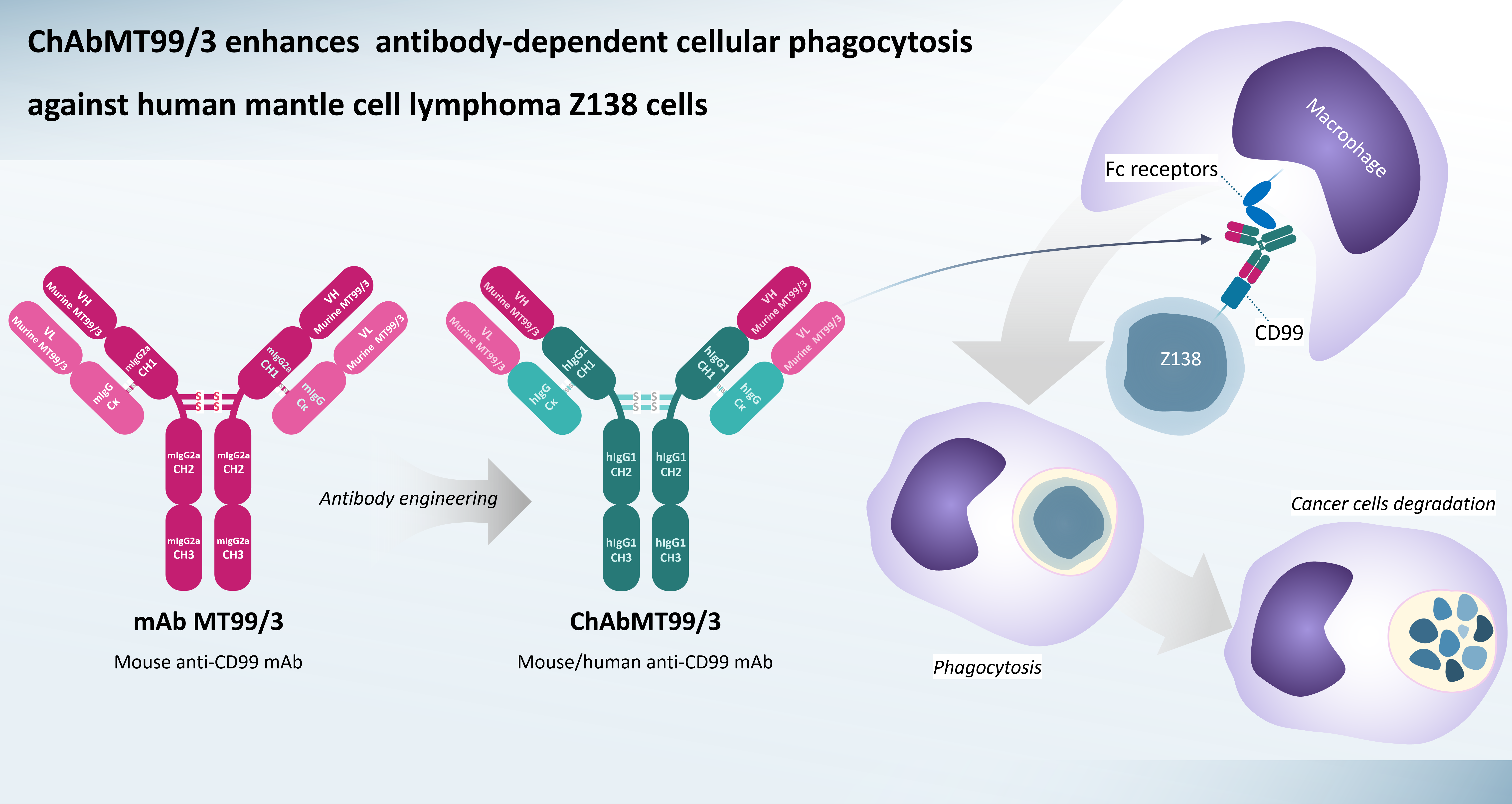
Background: Mantle cell lymphoma (MCL) is an aggressive form of B-cell nonHodgkin lymphoma. The elimination of MCL cells via phagocytosis is essential for cancer eradication. Therefore, discovering novel targeted antibodies that can induce phagocytosis is needed. We have demonstrated that our in-houseproduced mouse anti-CD99 mAb clone MT99/3 could induce potent anticancer activities against MCL cell lines in both in vitro and in vivo mouse xenograft models. Nevertheless, for use in humans, the mouse mAb needs to be transformed into a mouse/human chimeric mAb that contains a human Fc region to activate human immune effector functions, especially macrophage-mediated phagocytosis. Antibody-dependent cellular phagocytosis (ADCP) mediated by mouse/human chimeric mAb MT99/3 against MCL has not been previously reported.
Objective: This study aimed to genetically engineer a mouse/human chimeric antibody against human CD99 derived from mouse mAb MT99/3 and to evaluate its effect in mediating the ADCP mechanism for eradicating MCL cells in vitro using monocyte-derived macrophages.
Materials and methods: The expression plasmid to produce chimeric anti-CD99 antibody, ChAbMT99/3, was constructed by fusing the variable domains of mouse mAb MT99/3 with the constant domains of human IgG1 and the constant domains of kappa light chain. ChAbMT99/3 was expressed in the stable human expression system based on HEK293T cells. ChAbMT99/3 was purified from the culture supernatant of ChAbMT99/3-expressing HEK293T cells using Protein G chromatography. The purity and structure of ChAbMT99/3 were verified by SDS-PAGE and western blotting. The binding specificity and activity were determined by staining with cells expressing recombinant and native human CD99. The anticancer activity of ChAbMT99/3 in mediating the ADCP mechanism against MCL cell line Z138 using human monocytederived macrophages was evaluated.
Results: We successfully constructed the plasmid to produce ChAbMT99/3. Human HEK293T cells stably expressing ChAbMT99/3 were established. The ChAbMT99/3- expressing HEK293T cells could secrete ChAbMT99/3 into the culture supernatant. The high purity and complete IgG structure of ChAbMT99/3 were obtained from the purification process. Crucially, this chimeric antibody retained its binding reactivity to recombinant and native human CD99. In addition, the produced ChAbMT99/3, upon binding to MCL cells, significantly enhanced ADCP against MCL cell line Z138 in a dose-dependent manner.
Conclusion: The production of a mouse/human chimeric antibody against human CD99 derived from mouse mAb MT99/3 was successful. The engineered antibody could mediate ADCP activity against MCL cells. The produced ChAbMT99/3 might be a promising therapeutic candidate for MCL treatment.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Campo E, Raffeld M, Jaffe ES. Mantle-cell lymphoma. Semin Hematol. 1999; 36(2): 115-27.
Dabaja B, Ha CS, Cox JD. Chapter 34 - Leukemias and Lymphomas. In: Cox JD, Ang KK, editors. Radiation Oncology (Ninth Edition). Philadelphia: Mosby; 2010. p. 875-911.
Hanel W, Epperla N. Emerging therapies in mantle cell lymphoma. J Hematol Oncol. 2020; 13(1): 79. doi: 10.1186/s13045-020-00914-1
Fischer L, Jiang L, Bittenbring JT, Huebel K, Schmidt C, Duell J, et al. The addition of rituximab to chemotherapy improves overall survival in mantle cell lymphoma-a pooled trials analysis. Ann Hematol. 2023; 102(10): 2791-801. doi: 10.1007/s00277-023-05385-1
Roué G, Sola B. Management of Drug Resistance in Mantle Cell Lymphoma. Cancers (Basel). 2020; 12(6): 1565. doi: 10.3390/cancers12061565
Zahavi D, Weiner L. Monoclonal Antibodies in Cancer Therapy. Antibodies (Basel). 2020; 9(3): 34. doi: 10.3390/antib9030034
Grossbard ML, Press OW, Appelbaum FR, Bernstein ID, Nadler LM. Monoclonal Antibody-Based Therapies of Leukemia and Lymphoma. Blood. 1992; 80(4): 863- 78. doi: 10.1182/blood.V80.4.863.863
Cao X, Chen J, Li B, Dang J, Zhang W, Zhong X, et al. Promoting antibody-dependent cellular phagocytosis for effective macrophage-based cancer immunotherapy. Sci Adv. 2022; 8(11): eabl9171. doi: 10.1126/sciadv.abl9171
Kamen L, Myneni S, Langsdorf C, Kho E, Ordonia B, Thakurta T, et al. A novel method for determining antibody-dependent cellular phagocytosis. J Immunol Methods. 2019; 468: 55-60. doi: 10.1016/j.jim.2019. 03.001
Van Wagoner CM, Rivera-Escalera F, Jaimes-Delgadillo NC, Chu CC, Zent CS, Elliott MR. Antibody-mediated phagocytosis in cancer immunotherapy. Immunological Reviews. 2023; 319(1): 128-41. doi: 10.1111/imr.13265
Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell. 2010; 142(5): 699- 713. doi: 10.1016/j.cell.2010.07.044
Takheaw N, Sittithumcharee G, Kariya R, Kasinrerk W, Okada S. Anti-human CD99 antibody exerts potent antitumor effects in mantle cell lymphoma. Cancer Immunol Immunother. 2021; 70(6): 1557-67. doi: 10.1007/s00262-020-02789-0
Carter P. Improving the efficacy of antibody-based cancer therapies. Nature Reviews Cancer. 2001; 1(2): 118-29. doi: 10.1038/35101072
Khan FH. Chapter 25 - Antibodies and Their Applications. In: Verma AS, Singh A, editors. Animal Biotechnology. San Diego: Academic Press; 2014. p. 473-90.
Kasinrerk W, Tokrasinwit N, Moonsom S, Stockinger H. CD99 monoclonal antibody induce homotypic adhesion of Jurkat cells through protein tyrosine kinase and protein kinase C-dependent pathway. Immunology Letters. 2000; 71(1): 33-41. doi: 10.1016/S0165-2478(99)00165-0
Takheaw N, Laopajon W, Chuensirikulchai K, Kasinrerk W, Pata S. Exploitation of human CD99 expressing mouse myeloma cells as immunogen for production of mouse specific polyclonal antibodies. Protein Expr Purif. 2017; 134: 82-8. doi: 10.1016/j.pep.2017.02.015
Takheaw N, Kotemul K, Chaiwut R, Pata S, Laopajon W, Rangnoi K, et al. Transcriptome Analysis Reveals the Induction of Apoptosis-Related Genes by a Monoclonal Antibody against a New Epitope of CD99 on T-Acute Lymphoblastic Leukemia. Antibodies. 2024; 13(2): 42. doi: 10.3390/antib13020042
Shi J, Zhang Z, Cen H, Wu H, Zhang S, Liu J, et al. CAR T cells targeting CD99 as an approach to eradicate T-cell acute lymphoblastic leukemia without normal blood cells toxicity. Journal of Hematology & Oncology. 2021; 14(1): 162. doi: 10.1186/s13045-021-01178-z
Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Molecular Immunology. 2007; 44(16): 3823-37. doi: 10.1016/j.molimm.2007.06.151
Overdijk MB, Verploegen S, Bögels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, et al. Antibodymediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015; 7(2): 311-21. doi: 10.1080/19420862.2015.1007813
Beers SA, French RR, Chan HT, Lim SH, Jarrett TC, Vidal RM, et al. Antigenic modulation limits the efficacy of anti-CD20 antibodies: implications for antibody selection. Blood. 2010; 115(25): 5191-201. doi: 10.1182/blood-2010-01-263533
Doevendans E, Schellekens H. Immunogenicity of Innovative and Biosimilar Monoclonal Antibodies. Antibodies (Basel). 2019; 8(1): 21. doi: 10.3390/antib8010021
Yu J, Song Y, Tian W. How to select IgG subclasses in developing anti-tumor therapeutic antibodies. Journal of Hematology & Oncology. 2020; 13(1): 45. doi: 10. 1186/s13045-020-00876-4
Kretschmer A, Schwanbeck R, Valerius T, Rösner T. Antibody Isotypes for Tumor Immunotherapy. Transfus Med Hemother. 2017; 44(5): 320-6. doi: 10.1159/000479240
Wirt T, Rosskopf S, Rösner T, Eichholz KM, Kahrs A, Lutz S, et al. An Fc Double-Engineered CD20 Antibody with Enhanced Ability to Trigger Complement Dependent Cytotoxicity and Antibody-Dependent Cell-Mediated Cytotoxicity. Transfus Med Hemother. 2017; 44(5): 292-300. doi: 10.1159/000479978
Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006; 6(5): 343-57. doi: 10.1038/nri1837
Dumont J, Euwart D, Mei B, Estes S, Kshirsagar R. Human cell lines for biopharmaceutical manufacturing: history, status, and future perspectives. Crit Rev Biotechnol. 2016; 36(6): 1110-22. doi: 10.3109/07388551.2015.1084266
Tan E, Chin CSH, Lim ZFS, Ng SK. HEK293 Cell Line as a Platform to Produce Recombinant Proteins and Viral Vectors. Front Bioeng Biotechnol. 2021; 9: 796991. doi: 10.3389/fbioe.2021.796991
Abaandou L, Quan D, Shiloach J. Affecting HEK293 Cell Growth and Production Performance by Modifying the Expression of Specific Genes. Cells. 2021; 10(7): 1667. doi: 10.3390/cells10071667
Aroldi A, Mauri M, Ramazzotti D, Villa M, Malighetti F, Crippa V, et al. Effects of blocking CD24 and CD47 ‘don’t eat me’ signals in combination with rituximab in mantle-cell lymphoma and chronic lymphocytic leukaemia. J Cell Mol Med. 2023; 27(20): 3053-64. doi: 10.1111/jcmm.17868
Khalaji A, Yancheshmeh FB, Farham F, Khorram A, Sheshbolouki S, Zokaei M, et al. Don’t eat me/eat me signals as a novel strategy in cancer immunotherapy. Heliyon. 2023; 9(10): e20507. doi: 10.1016/j.heliyon.2023.e20507
Liu Ye, Wang Y, Yang Y, Weng L, Wu Q, Zhang J, et al. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduction and Targeted Therapy. 2023; 8(1): 104. doi: 10.1038/s41392-023-01365-z
Liao-Chan S, Daine-Matsuoka B, Heald N, Wong T, Lin T, Cai AG, et al. Quantitative assessment of antibody internalization with novel monoclonal antibodies against Alexa fluorophores. PLoS One. 2015; 10(4): e 0124708. doi: 10.1371/journal.pone.0124708
Jin H, D’Urso V, Neuteboom B, McKenna SD, Schweickhardt R, Gross AW, et al. Avelumab internalization by human circulating immune cells is mediated by both Fc gamma receptor and PD-L1 binding. OncoImmunology. 2021; 10(1): 1958590. doi: 10.1080/2162402X.2021.1958590