Review article: An overview of exosomes in biology and their potential applications in regenerative medicine
Main Article Content
Abstract
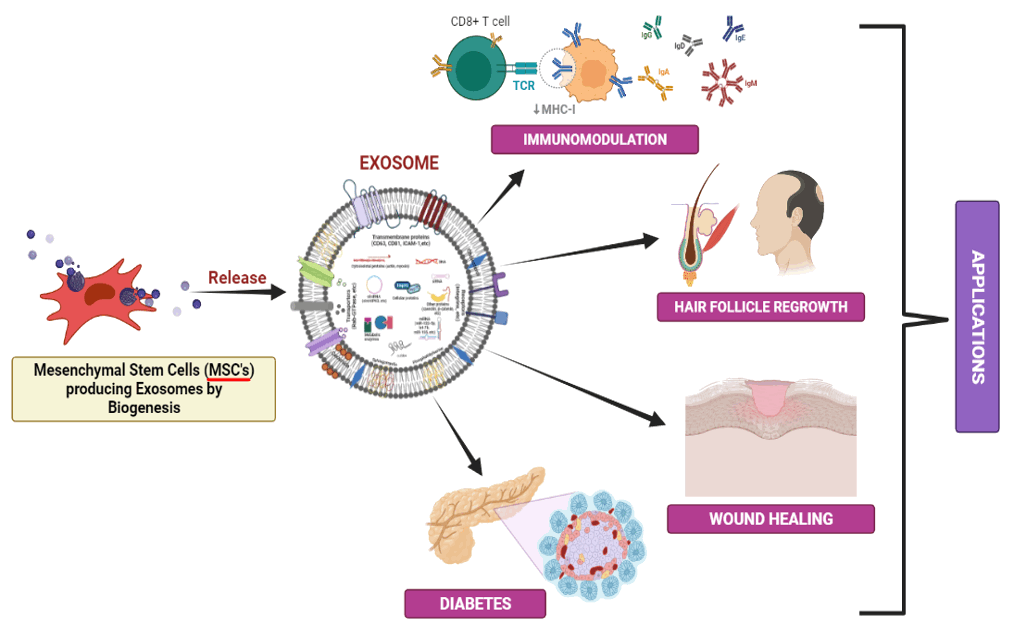
Extracellular vesicles (EVs), commonly acknowledged as Exosomes, are tiny, single-membrane, secreted organelles that range in size from 40 to 150 nm. They are noticeably abundant in various proteins, lipids, nucleic acids, and glycoconjugates and share the same structure as cells. Numerous and non-hematopoietic cell types continuously manufacture and release stable, less toxic, and biocompatible exosomes with many complex compounds (in the form of various signaling molecules, miRNA, and mRNA) in the liquid parts of the body. Exosomes help in intercellular communication/transfer of proteins, RNA, cell differentiation, immune signaling, delivering antigens, angiogenesis, and stress response. In recent studies, researchers found that Mesenchymal Stem cells (MSCs) generate exosomes that symbolize biological processes like tissue regeneration by encasing and delivering active biomolecular species to the infected/damaged cells and tissues. The most extensive research in regenerative medicine has focused on MSCs-Exosomes. Regenerative medicine plays a crucial role in restoring the damaged/lost parts of organs and tissues and aiding in wound healing. Immunomodulation and tissue repair are possible by introducing Mesenchymal Stem cell (MSC) exosomes, which have triggered remodeling reactions. They produce local anti-inflammatory and healing signals crucial for regeneration and tissue repair. The primary goal of this review is to highlight the MSCs-exosome’s mechanism of action and its therapeutic uses in clinical settings. Also, it highlights new developments in employing MSCsexosomes to treat various ailments and disorders.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Jacques G, et Luc Sensébé. Mesenchymal stromal cells: Clinical challenges and therapeutic Opportunities. Cell Stem Cell. 2018; 22(6): 824-33. doi.org/10.1016/j.stem.2018.05.004.
Tiziana S, et al. Clinical trials with mesenchymal stem cells: An update. Cell Transplantation. 2016; 25(5): 829-48. doi.org/10.3727/096368915X689622.
Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells
(MSCs): a comparison of adult and neonatal tissuederived MSCs. Cell Commun Signal. 2011; 9 (1): 12.
Maqsood M, et al. Adult mesenchymal stem cells and their exosomes: Sources, characteristics, and application in regenerative medicine. Life Sciences. 2020; 256: 118002. doi.org/10.1016/j.lfs.2020.118002.
Yuanxia Z, et al. Mesenchymal stem cell-derived extracellular vesicles/exosome: A promising therapeutic strategy for Intracerebral hemorrhage. Regen Ther. 2023; 22: 181-90. doi.org/10.1016/j.reth.2023.01.006.
He L, et al. Bone marrow mesenchymal stem cellderived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020; 11(1): 276. doi: 10.1186/s13287-020-01781-w.
Li T, et al. Mesenchymal stem cell-derived exosomal microRNA-3940-5p inhibits colorectal cancer metastasis by targeting integrin α6. Dig Dis Sci. 2021; 66(6): 1916-27. doi: 10.1007/s10620-020-06458-1.
Gillian C, et al. Exosomes and other extracellular vesicles: The new communicators in parasite infections. Trends Parasitol. 2015; 31(10): 477-89. doi.org (Crossref). doi.org/10.1016/j.pt.2015.06.009.
Peter W. The nature and significance of platelet products in human plasma. British J Haematol. 1967; 13(3): 269-88. doi.org/10.1111/j.1365-2141.1967.tb08741.x.
Johnstone RM, et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987; 262(19): 9412-20. doi.org/10.1016/S0021-9258(18)48095-7.
Abbaszadeh H, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles: a novel therapeutic paradigm. J Cell Physiol. 2020; 235(2): 706-17. doi: 10.1002/jcp.29004.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J Extracell Vesicles. 2018: 23; 7(1):
doi: 10.1080/20013078.2018.1535750.
Lötvall J, Hill AF, Hochberg F et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014; 22(3): 26913. doi.org/10.3402/jev.v3.26913.
Pap E, et al. Highlights of a New Type of Intercellular Communication: Microvesicle-Based Information Transfer. Inflamm Res. 2009; 58(1): 1-8. doi.org/10.1007/s00011-008-8210-7.
Sonam G, et al. The exosome journey: From biogenesis to uptake and intracellular signaling. Cell Commun Signal. 2021; 19(1): 47. doi.org/10.1186/s12964-021-00730-1.
Szabo G, Momen-Heravi F. Extracellular vesicles and exosomes: biology and pathobiology. The Liver: Biology and pathobiology. Wiley, New York, 2020, pp 1022-7. doi.org/10.1002/9781119436812.ch78
Antonelou MH, Seghatchian J. Update on extracellular vesicles inside red blood cell storage units: adjust the sails closer to the new wind. Transfus Apher Sci. 2016; 55(1): 92-104. doi: 10.1016/j.transci.2016.07.016.
Michelle M.and Leonard J. FedExosomes: Engineering therapeutic biological nanoparticles that truly deliver. Pharmaceuticals. 2013; 6(5): 659-80. doi.org/10.3390/ph6050659.
Pluchino S. and Smith JA. Explicating exosomes: reclassifying the rising stars of intercellular communication. Cell. 2019; 177(2): 225-7. doi: 10.1016/j.cell.2019.03.020.
Mathivanan S, Fahner CJ, Reid GE, and Simpson RJ. ExoCarta. Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012; 40(D1): D1241-4, 2012. doi: 10.1093/nar/gkr828.
Simpson RJ, Lim JWE, Moritz RL, and Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2014; 6(3): 267-83. doi: 10.1586/epr.09.17.
Munir H, Ward LSC, McGettrick HM. Mesenchymal stem cells as endogenous regulators of inflammation in stromal immunology. Adv Exp Med Biol. 2018; 1060: 73-98. doi: 10.1007/978-3-319-78127-3_5.
Tanaka Y, Kamohara H, Kinoshita K, et al. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer. 2013; 119(6): 1159-67. doi: 10.1002/cncr.27895.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200(4): 373-83. doi: 10.1083/jcb.201211138.
Edwin van der Pol, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacological Reviews. 2012; 64(3): 676-705. doi: 10.1124/pr.112. 005983.
Lee TH, D’Asti E, Magnus N, Al-Nedawi K, Meehan B, and Rak J. Microvesicles as mediators of intercellular communication in cancer—the emerging science of cellular ‘debris’. Semin Immunopathol. 2011; 33(5): 455-67. doi 10.1007/s00281-011-0250-3 10.
Corbeil D, Marzesco AM, Wilsch-Brauninger M, Huttner WB. (2010). The intriguing links between prominin-1 (CD133), cholesterol-based membrane microdomains, remodeling of apical plasma membrane protrusions, extracellular membrane particles, and (neuro)epithelial cell differentiation. FEBS Letters. 2010; 584(9): 1659- 64. doi: 10.1016/j.febslet.2010.01.050.
Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011; 6(4): 481-92. doi: 10.2217/rme.11.35.
Yuan Z, et al. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019; 9(1): 19. doi. org/10.1186/s13578-019-0282-2.
Clifford V. Harding, John E. Heuser, Philip D. Stahl. Exosomes: Looking back three decades and into the
future. J Cell Biol. 2013; 200(4): 367-71. doi: 10.1083/jcb.201212113.
Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983; 97, 329-39. doi: 10.1083/jcb.97.2.329.
Pan BT, Johnstone RM. (1983). Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell. 1983; 33: 967-78. doi: 10.1016/0092-8674(83) 90040-5.
Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: Introducing the next small big thing. International Journal of Molecular Sciences. 2016; 17: 1-30. doi: 10.3390/ijms17020170.
Volker S, Barz D. Characterization of Cellular and Extracellular Plasmamembrane Vesicles from a Low Metastatic Lymphoma (Eb) and Its High Metastatic Variant (ESb): Inhibitory Capacity in Cell-Cell Interaction Systems. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1986; 860(2): 236-42. doi.org/10.1016/0005-2736(86)90519-5.
Pan, B. T., & Johnstone, R. M. (1983). Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell, 33, 967–978
Johnstone RM, Mathew A, Mason AB, Teng K. Exosome formation during maturation of mammalian and avian reticulocytes: Evidence that exosome release is a major route for externalization of obsolete membrane proteins. Journal of Cellular Physiology. 1991; 147: 27-36. doi.org/10.1002/jcp.1041470105.
Yin K, Wan, S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomark Res. 2019; 7: 1-8. doi.org/10.1186/s40364-019-0159-x.
Harrell CR, et al. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 2019; 8(12): 1605. doi: 10.3390/cells8121605.
Lamparski HG, Metha-Damani A, Yao J-Y, Patel S, Hsu D-H, Ruegg C, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods (2002) 270(2):211–26. doi: 10.1016/s0022-1759(02)00330-7.
Min L, Zhu S, Chen L, Liu X, Wei R, Zhao L, et al. Evaluation of circulating small extracellular vesicles derived miRNAs as biomarkers of early colon cancer: A comparison with plasma total miRNAs. J Extracel Vesicles. 2019; 8: 1643670. doi: 10.1080/20013078.2019.1643670.
Wennink JWH, Liu Y, Mäkinen PI, et al. Macrophage selective photodynamic therapy by meta-tetra (hydroxyphenyl)chlorin loaded polymeric micelles: a possible treatment for cardiovascular diseases. Eur J Pharm Sci. 2017; 107 :112-125. doi: 10.1016/j.ejps.2017.06.038.
Pala RR, Anju VT, Dyavaiah M, Busi S, Nauli SM. Nanoparticle-mediated drug delivery for the treatment of cardiovascular diseases. Int J Nanomedicine. 2020: 15: 3741-69. doi: 10.2147/IJN.S250872
Wang R, Ding Q, Yaqoob U, et al. Exosome adherence and internalization by hepatic stellate cells triggers Sphingosine 1-phosphate-dependent migration. J Biol Chem. 2015; 290: 30684-96. doi: 10.1074/jbc.M115.671735.
Tickner JA, Urquhart AJ, Stephenson SA, Richard DJ, O’Byrne KJ. Functions and therapeutic roles of exosomes in cancer. Front Oncol. 2014; 4: 127. doi: 10.3389/fonc.2014.00127.
Frydrychowicz Kolecka-Bednarczyk MA, Madejczyk M, Yasar MS, Dworacki G. Exosomes-structure, biogenesis and biological role in non-small-cell lung cancer. Scand J Immunol. 2015; 81(1): 2-10. doi: 10.1111/sji.12247.
Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007; 89: 205-12. doi: 10.1016/j.biochi.2006.10.014.
Pfeffer SR. Two Rabs for exosome release. Nat Cell Biol. 2010; 12(1): 3-4. doi: 10.1038/ncb0110-3.
Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q, Formation and release of arrestin domain-containing protein 1- mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci. 2012; 109(11): 4146-51. doi: 10.1073/pnas.1200448109.
Lou G, Chen GZ, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Ex Mo Med. 2017; 49(6): e346.
Schey KL, Luther JM, Rose KL. Proteomics characterization of exosome cargo. Methods. 2015; 87: 75-82. doi: 10.1016/j.ymeth.2015.03.018.
Tai YL, Chen KC, Hsieh JT, Shen TL. Exosomes in cancer development and clinical applications. Cancer Sci. 2018; 109(8): 2364-74. doi: 10.1111/cas.13697
Zhang H, Freitas D, Kim HS, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018; 20(3): 332-43. doi: 10.1038/s41556-018-0040-4.
Cosenza S, Toupet K, Maumus M, et al. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018; 8(5): 1399-410. doi: 10.7150/thno.21072.
K. Yin, S. Wang, and R. C. Zhao, “Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm,” Biomarker Research. 2019; 7(1): 8.
Xie Y, Dang W, Zhang S, et al. The role of exosomal noncoding RNAs in cancer. Mol Cancer. 2019; 18: 37.
Li SP, Lin ZX, Jiang XY, Yu XY. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol Sin. 2018; 39: 542-51.
Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cellbased therapy. CMLS. 2020; 77(14): 2771-94. doi: 10.1007/s00018-020-03454-6.
Mikael S, Raposo G. Exosomes - Vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009; 21(4): 575-81. doi.org/10.1016/j.ceb.2009.03.007.
Williams RL, Urbé S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007; 8(5): 355-68. doi.org/10.1038/nrm2162.
Jatta H, Helenius A. Endosome maturation: Endosome maturation. EMBO J. 2011; 30(17): 3481-500. doi. org/10.1038/emboj.2011.286.
Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, and Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019; 18: 75.
Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984; 35: 256-63
Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev. Cell. 2011; 21: 77-91. doi: 10.1016/j.devcel.2011. 05.015.
Hirano S, Kawasaki M, Ura H, Kato R, Raiborg C, Stenmark H, et al. Double-sided ubiquitin binding of Hrs-UIM in endosomal protein sorting. Nat Struct Mol Biol 2006; 13: 272-7. doi: 10.1038/nsmb1051.
Babst M, Sato TK, Banta LM, Emr SD. Endosomal transport function in yeast requires a novel AAAtype ATPase, Vps4p. EMBO J. 1997; 16: 1820-31. doi: 10.1093/emboj/16.8.1820.
Thomas J, Fürthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol. 2018; 74: 66-77. doi.org/10.1016/j.semcdb. 2017.08.022.
Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001; 106: 145-55. doi: 10.1016/s0092-8674(01)00434-2.
Misra S. Hurley JH. Crystal structure of a phosphatidylinositol 3-phosphate-specific membranetargeting motif, the FYVE domain of Vps27p. Cell. 1999; 97: 657-66. doi: 10.1016/s0092-8674(00)80776-x.
Raiborg C, Bremnes B, Mehlum A, Gillooly DJ, D’Arrigo A, Stang E, et al. FYVE and coiled-coil domains determine the specific localization of Hrs to early endosomes. J Cell Sci. 2001b; 114: 2255-63. doi: 10.1242/jcs.114. 12.2255.
Stahelin RV, Long F, Diraviyam K, Bruzik KS, Murray D, Cho W. Phosphatidylinositol 3-phosphate induces the membrane penetration of the FYVE domains of Vps27p and Hrs. J Biol Chem. 2002; 277: 26379-88. doi: 10.1074/jbc.M201106200.
Raiborg C, Bache KG, Mehlum A, Stang E, Stenmark H. Hrs recruits clathrin to early endosomes. EMBO J. 2001a; 20: 5008-21. doi: 10.1093/emboj/20.17.5008.
Raiborg C, Bache KG, Gillooly DJ, Madshus IH, Stang E, Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 2002; 4: 394-8. doi: 10.1038/ncb791
Raiborg C, Wesche J, Malerod L, Stenmark H. Flat clathrin coats on endosomes mediate degradative protein sorting by scaffolding Hrs in dynamic microdomains. J Cell Sci.2006; 119: 2414-24. doi: 10.1242/jcs.02978.
Hurley JH. The ESCRT Complexes. Crit Rev Biochem Mol Biol. 2010; 45(6): 463-87. doi.org/10.3109/10409238.2010.502516.
Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009; 458(7237): 445-52. doi.org/10.1038/nature07961.
Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell. 2002; 3: 271-82. doi: 10.1016/s1534-5807(02)00220-4.
Chiaruttini N, Redondo-Morata L, Colom A, Humbert F, Lenz M, Scheuring S. Relaxation of loaded ESCRT-III spiral springs drives membrane deformation. Cell. 2015; 163: 866-79. doi: 10.1016/j.cell.2015.10.017.
Henne WM, Stenmark H, Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol. 2013; 5: a016766. doi: 10.1101/cshperspect.a016766
Agromayor M, Martin-Serrano J. Interaction of AMSH with ESCRT-III and deubiquitination of endosomal cargo. J Biol Chem. 2006; 281: 23083-91.
Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol. 2008; 20: 4-11. doi: 10.1016/j.ceb.2007.12.002.
Dumas JJ, Merithew E, Sudharshan E, Rajamani D, Hayes S, Lawe D, et al. Multivalent endosome targeting by homodimeric EEA1. Mol Cell. 2001; 8(5): 947-58. doi.org/10.1016/S1097-2765(01)00385-9.
Janas T, et al. Mechanisms of RNA Loading into Exosomes. FEBS Lett. 2015; 589: 1391-8. doi.org/10.1016/j.febslet.2015.04.036.
Santosh P, et al. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 2014; 281: 2214-27. doi.org/10.1111/febs.12775.
Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003; 19: 397-422. doi: 10.1146/annurev. cellbio.19.111301.153609.
Kajimoto T, Okada T, Miya S, et al. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun. 2013; 4: 2712. doi: 10.1038/ncomms 3712.
Stuffers S, et al. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009; 10(7): 925- 37. doi.org/10.1111/j.1600-0854.2009.00920.x.
Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012; 14: 677-85.
Thompson CA, Purushothaman A, Ramani VC,
Vlodavsky I, Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem 2013; 288: 10093-9. doi: 10.1074/jbc.C112.444562.
Colombo M, Moita C, Van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013; 126: 5553-65. doi: 10.1242/jcs.128 868.
Tamai K, Tanaka N, Nakano T, Kakazu E, Kondo Y, Inoue J, et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem. Biophys Res. Commun. 2010; 399: 384-90. doi: 10.1016/j.bbrc.2010.07.083.
Razi M, Futter CE. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol Biol Cell. 2006; 17: 3469-83. doi: 10.1091/mbc.e05-11-1054.
Baietti M.F., Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, et al.(2012). Syndecansyntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677-85
Banfer S, Schneider D, Dewes J, Strauss MT, Freibert SA, Heimerl T, et al. Molecular mechanism to recruit galectin-3 into multivesicular bodies for polarized exosomal secretion. Proc Natl Acad Sci USA. 2018; 115: E4396-E4405. doi: 10.1073/pnas.1718921115.
Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012; 28: 337- 62. doi: 10.1146/annurev-cellbio-092910-154152.
Larios J, Mercier V, Roux A, Gruenberg J. ALIX- and ESCRT-III dependent sorting of tetraspanins to exosomes. J Cell Biol. 2020, 219(3): e201904113. doi.org/10.1083/jcb.201904113.
Campsteijn C, Vietri M, Stenmark H. Novel ESCRT functions in cell biology: Spiraling out of control? Curr Opin Cell Biol. 2016; 41: 1-8. doi.org/10.1016/j.ceb.2016.03.008.
Petsalaki E, Zachos G. Clks 1, 2 and 4 prevent chromatin breakage by regulating the Aurora B-dependent abscission checkpoint. Nat Commun. 2016; 7: 11451. doi.org/10.1038/ncomms11451.
Wei D, Zhan W, Gao Y, Huang L, Gong R, Wang W, et al. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021; 31: 157-77.
Stuffers S, Wegner CS, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009; 10: 925-37. doi: 10.1111/j.1600-0854.2009.00920.x.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008; 29; 319(5867): 1244-7.doi: 10.1126/science.1153124.
Mazzeo C, Calvo V, Alonso R, Merida I, Izquierdo M. Protein kinase D1/2 is involved in the maturation of multivesicular bodies and secretion of exosomes in T and B lymphocytes. Cell Death Differ. 2016: 23, 99- 109. doi: 10.1038/cdd.2015.72.
Laulagnier K, Grand D, Dujardin A, Hamdi S, VincentSchneider H, Lankar D, et al. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett. 2004; 572: 11-14. doi: 10.1016/j.febslet.2004.06.082.
Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale N, et al. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014; 5: 3477. doi: 10.1038/ncomms447.
Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: A novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010; 190: 1079-91. doi: 10.1083/jcb.201002049.
Van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, et al. The tetraspanin CD63 regulates ESCRT independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011; 21: 708-21.
Hurwitz SN, Nkosi D, Conlon MM, York SB, Liu X, Tremblay DC, et al. CD63 regulates Epstein-Barr virus LMP1 exosomal packaging, enhancement of vesicle production, and noncanonical NF-kappaB signaling. J Virol. 2017; 91(5): e02251-16. doi: 10.1128/JVI.02251-16.
Edgar JR, Manna PT, Nishimura S, Banting G, Robinson MS. Tetherin is an exosomal tether. Elife 2016;5: e17180. doi.org/10.7554/eLife.17180.
Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci. 2002; 115: 2505-15. doi: 10.1242/jcs.115.12.2505.
Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005; 6: 131-43. doi: 10.1111/j.1600-0854.2004.00257.x.
Messenger SW, Woo SS, Sun ZZ, Martin TFJ. A Ca2+-stimulated exosome release pathway in cancer cells is regulated by Munc13-4. J Cell Biol. 2019; 218: 1422. doi: 10.1083/jcb.201710132.
Ruiz-Martinez M, Navarro A, Marrades RM, Vinolas N, Santasusagna S, Munoz C, et al. YKT6 expression, exosome release, and survival in non-small cell lung cancer. Oncotarget. 2016; 7: 51515-24. doi: 10.18632/oncotarget.9862.
Hyenne V, Apaydin A, Rodriguez D, Spiegelhalter C, Hoff-Yoessle S, Diem M, et al. RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol. 2015; 211: 27-37. doi: 10.1083/jcb.201504136.
Fader CM, Sanchez DG, Mestre MB, Colombo MI. TIVAMP/VAMP7 and VAMP3/cellubrevin: Two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta. 2009; 1793: 1901-16. doi: 10.1016/j.bbamcr.2009.09.011.
Ostrowski M, Carmo NB, Krumeich, S Fange I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway.
Nat Cell Biol. 2010; 12: 19-30. doi: 10.1038/ncb2000.
Zhao R, Chen X, Song H, Bie B, Zhang B. Dual role of MSCs-derived exosomes in tumor development. Stem Cells Intl. 2020; 2020: 88447302020 doi: 10. 1155/2020/8844730.
Tschuschke M, Kocherova I, Bryja A, Mozdziak P, Volponi AA, Janowicz K, et al. Inclusion biogenesis, methods of isolation and clinical application of human cellular exosomes. J Clin Med. 2020; 9(2): 436. doi.org/10.3390/jcm9020436.
Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007; 450(7168); 435-9. doi: 10.1038/nature06307.
Simons M, Raposo G. Exosomes-vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009; 21: 575-81. doi.org/10.1016/j.ceb.2009.03.007.
Mathivanan S, Lim JW, Tauro BJ, Ji H, Moritz RL, Simpson RJ. Proteomics analysis of A33 immunoaffinitypurified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics. 2010; 9(2): 197-208. doi: 10.1074/mcp.M900152-MCP200
Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010; 11: 675-87. doi.org/10.1111/j.1600-0854.2010.01041.
Wang M, Yuan Q, Xie L. Mesenchymal stem cellbased immunomodulation: properties and clinical application. Stem Cells Int. 2018; 2018: 3057624. doi: 10.1155/2018/3057624.
Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016; 7: e2062. doi: 10.1038/cddis.2015.327.
Gomzikova MO, James V, Rizvanov AA. Therapeutic application of mesenchymal stem cells derived extracellular vesicles for immunomodulation. Front Immunol. 2019; 10: article 2663. doi.org/10.3389/fimmu.2019.02663
Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014; 23: 123-44. doi: 10.1089/scd.2013.0479.
Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017; 196:1275-86. doi: 10.1164/rccm.201701-0170OC.
Van den Akker F, Vrijsen KR, Deddens JC, Buikema JW, Mokry M, van Laake LW, et al. Suppression of T cells by mesenchymal and cardiac progenitor cells is partly mediated via extracellular vesicles. Heliyon. 2018; 4: e00642. doi: 10.1016/j.heliyon.2018.e00642.
Casado JG, Blazquez R, Vela FJ, Alvarez V, Tarazona R, Sanchez-Margallo FM. Mesenchymal stem cellderived exosomes: immunomodulatory evaluation in an antigen-induced synovitis porcine model. Front Vet Sci. 2017; 4: 39. doi: 10.3389/fvets.2017.00039.
Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, et al. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015; 192: 324-6. doi: 10.1164/rccm.201410-1765OC.
Shigemoto-Kuroda T, Oh JY, Kim DK, Jeong HJ, Park SY, Lee HJ, et al. MSCs-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Rep. 2017; 8: 1214-25. doi: 10.1016/j.stemcr.2017.04.008.
Alzahrani FA, Saadeldin IM, Ahmad A, Kumar D, Azhar EI, Siddiqui AJ, et al. The potential use of mesenchymal stem cells and their derived exosomes as immunomodulatory agents for COVID-19 patients. Stem Cells Int. 2020; 2020: 8835986. doi: 10.1155/2020/8835986
Carrasco E, Soto-Heredero G, Mittelbrunn M. The role of extracellular vesicles in cutaneous remodeling and hair follicle dynamics. Int J Mol Sci. 2019; 20(11): 2758. doi: 10.3390/ijms20112758.Solanas G,
Solanas G, Benitah SA. Regenerating the Skin: A task for the heterogeneous stem cell pool and surrounding niche. Nat Rev Mol Cell Biol. 2013; 14: 737-48. doi: 10.1038/nrm3675.
Wang B, Liu XM, Liu ZN, Wang Y, Han X, Lian AB, et al. Human hair follicle-derived mesenchymal stem cells: Isolation, expansion, and differentiation. World J Stem Cells. 2020; 12(6): 462-70. doi: 10.4252/wjsc.v12.i6.462.
Millar SE. Molecular mechanisms regulating hair follicle development. J Investig Dermatol. 2002; 118: 216-25. doi: 10.1046/j.0022-202x.2001.01670.x.
Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012; 14: 1036-45. doi: 10.1038/ncb2574.
McBride JD, Rodriguez-Menocal L, Guzman W, Candanedo A, Garcia-Contreras M, Badiavas EV. Bone marrow mesenchymal stem cell-derived CD63+ exosomes transport Wnt3a exteriorly and enhance dermal fibroblast proliferation, migration, and angiogenesis In vitro. Stem Cells Dev. 2017; 26: 1384-98. doi: 10.1089/scd.2017.0087.
Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMScs-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells .2015; 33: 2158-68. doi: 10.1002/stem.1771.
Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-Catenin pathway. Stem Cells Transl Med. 2015; 4: 513-22. doi: 10.5966/sctm.2014-0267.
Chen Q, Takada R, Noda C, Kobayashi S, Takada S. Different populations of Wnt-containing vesicles are individually released from polarized epithelial cells. Sci Rep. 2016; 6: 35562. doi: 10.1038/srep35562.
Rajendran RL, Gangadaran P, Bak SS, Oh JM,
Kalimuthu S, Lee HW, et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from Telogen to Anagen in mice. Sci Rep. 2017; 7: 155604. doi: 10.1038/s41598-017-15505-3.
Zhou L, Wang H, Jing J, Yu L, Wu X, Lu Z. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem Biophys Res Commun. 2018; 500(2): 325-32. doi: 10.1016/j. bbrc.2018.04.067.
Ahmed MI., Alam M, Emelianov VU, Poterlowicz K, Patel A, Sharov AA, et al. MicroRNA-214 controls skin and hair follicle development by modulating the activity of the Wnt pathway. J Cell Biol. 2014; 207: 549-67. doi: 10.1083/jcb.201404001.
Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, et al. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burninduced excessive inflammation. EBioMedicine. 2016; 8: 72-82. doi: 10.1016/j.ebiom.2016.04.030.
Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ. Chitosan wound dressings incorporating exosomes derived from microRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl Med. 2017; 6: 736-47. doi: 10.5966/sctm.2016-0275.
Dong L, Hao H, Xia L, Liu J, Ti D, Tong C, et al. Treatment of MSCs with Wnt1a-conditioned medium activates DP cells and promotes hair follicle regrowth. Sci Rep. 2014; 4: 5432. doi: 10.1038/srep05432.
Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt Proteins Are Secreted on Exosomes. Nat Cell Biol. 2012; 14: 1036-45.
Bian D, Wu Y, Song G, Azizi R, Zamani A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in Skin wound healing: A comprehensive review. Stem Cell Res Ther. 2022; 13(1): 24. doi.org/10.1186/s13287-021-02697-9.
Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, Conti B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics. 2020; 12(8): 735. doi: 10.3390/pharmaceutics12080735.
Frykberg Robert G. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015; 4(9): 560- 82. doi: 10.1089/wound.2015.0635.
Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010; 316(14): 2213-9. doi: 10.1016/j.yexcr.2010.05.009
Rani S, Ritter T. The exosome-A naturally secreted nanoparticle and its application to wound healing. Adv Mater. 2016; 28(27): 5542-52. doi: 10.1002/ adma.201504009.
Stephens P, Thomas DW. The cellular proliferative phase of the wound repair process. J Wound Care. 2002; 11(7): 253-61. doi: 10.12968/jowc.2002.11.7.26421.
Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015; 40: 82-8. doi: 10.1016/j.semcdb.2015.03.00125765629.
Hu P, Yang Q, Wang Q, Shi C, Wang D, Armato U, et al. Mesenchymal stromal cells-exosomes: A promising cell-free therapeutic tool for wound healing and cutaneous regeneration. Burns Trauma. 2019; 7: 38. doi: 10.1186/s41038-019-0178-8
Yang J, Liu XX, Fan H, Tang Q, Shou ZX, Zuo DM, et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One. 2015; 10: e014055 14607447. doi: 10.1371/journal.pone.0140551
Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016; 6: 32993. doi: 10.1038/srep32993.
Geiger A, Walker A, Nissen E. Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochem Biophys Res Commun. 2015; 467: 303-9. doi: 10.1016/j.bbrc.2015.09.16626454169.
Zhao B, Zhang Y, Han S, Zhang W, Zhou Q, Guan H et al. Exosomes derived from human amniotic epithelial cells accelerate wound healing and inhibit scar formation. J Mol Histol. 2017; 48: 121-32. doi: 10.1007/s10735-017-9711-x28229263.
Kou X, Xingtian Xu X, Chen C, Sanmillan ML, Cai T, Zhou Y. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Science Translational Medicine. 2018; 10(432): eaai8524. doi: 10.1126/scitranslmed.aai8524.
Marofi F, Alexandrovna KI, Margiana R, Bahramali M, Suksatan W, Abdelbasset WK, et al. MSCs and their exosomes: A rapidly evolving approach in the context of cutaneous wound therapy. Stem Cell Res Ther. 2012; 12(1): 597. doi.org/10.1186/s13287-021-02662-6.
He X, Dong Z, Cao Y, Wang H, Liu S, Liao L, et al. MSCsderived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019; 2019: 7132708 doi: 10.1155/2019/7132708.
Xiong J, Hu H, Guo R, Wang H, Jiang H. Mesenchymal stem cell exosomes as a new strategy for the treatment of diabetes complications. Front Endocrinol. 2021; 12: 646233. doi.org/10.3389/fendo.2021.646233.
Guay C, Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab. 2017; 19 Suppl 1: 137-46. doi: 10.1111/dom.13027.
Mahdipour E, Salmasi Z, Sabeti N. Potential of stem cell-derived exosomes to regenerate β Islets through Pdx-1 dependent mechanism in a rat Model of type 1 diabetes. J Cell Physiol. 2019; 234: 20310-21. doi: 10.1002/jcp.28631.
Nojehdehi S, Soudi S, Hesampour A, Rasouli S, Soleimani M, Hashemi SM. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem. 2018; 119: 9433-43. doi: 10.1002/jcb.27260.
Sabry D, Marzouk S, Zakaria R, Ibrahim HA, Samir M. The effect of exosomes derived from mesenchymal stem cells in the treatment of induced type 1 diabetes mellitus in rats. Biotechnol Lett. 2020; 42: 1597-610. doi: 10.1007/s10529-020-02908-y.
Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B, et al. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano. 2018; 12: 7613-28. doi: 10.1021/acsnano.7b07643.
Guay C, Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab. 2017; 19 Suppl 1: 137-46. doi: 10.1111/dom.13027.
Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019; 13: 555-68. doi: 10.1002/term.2799.
Rackov G, Garcia-Romero N, Esteban-Rubio S, Carrión-Navarro J, Belda-Iniesta C, Ayuso-Sacido A. vesicle-mediated control of cell function: The role of extracellular matrix and microenvironment. Front Physiol. 2018; 9: 651. doi: 10.3389/fphys.2018.00651.