Exploration of divalent metal transporter 1 (DMT1) gene intronic IVS4+44C/A polymorphisms in population exposed to cadmium Intronic IVS4+44C/A polymorphisms
Main Article Content
Abstract
Background: Cadmium exposure affects the expression of the DMT1 gene and the function of its transporter protein, impacting the transport and accumulation. This study investigates genetic polymorphisms to understand better the pivotal role of genetic factors in cadmium-related diseases within environmental health research.
Objective: The study sought to examine the intronic IVS4+44C/A polymorphism in the divalent metal transporter 1 (DMT1) gene among individuals aged 35-60 residing in regions contaminated with cadmium.
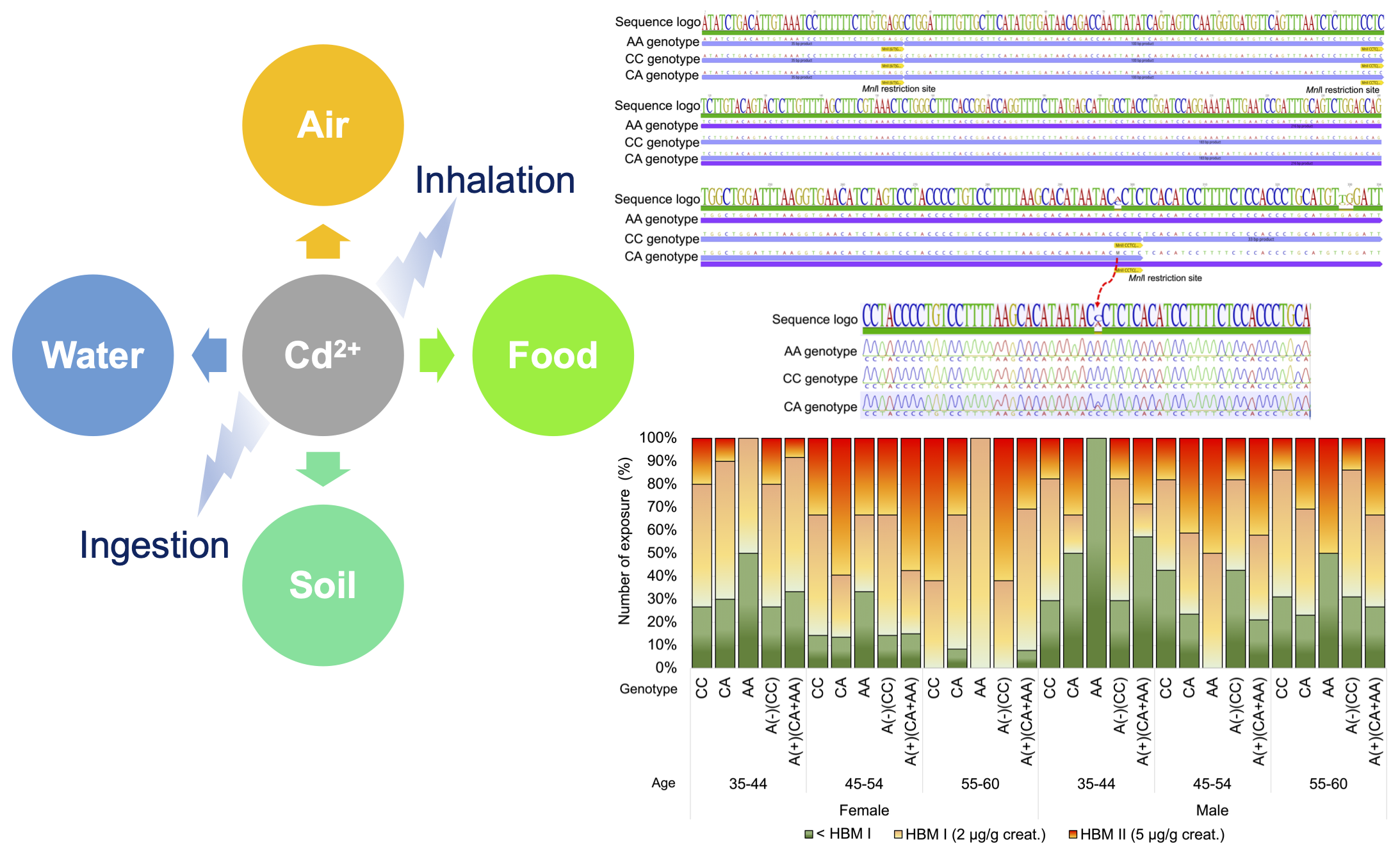
Materials and methods: Blood samples were collected from 306 genetically unrelated individuals (158 females and 148 males). The DMT1 IVS4+44C/A polymorphism was determined using restriction fragment length polymorphism (RFLP) and Sanger sequencing methods. Urinary cadmium levels were measured with graphite furnace atomic absorption spectrometry (GFAAS). Statistical analyses included Hardy-Weinberg equilibrium testing, analysis of variance (ANOVA), and student’s t-tests.
Results: The geometric mean of urinary cadmium levels were significantly higher in females (4.03±4.15 µg/gm creatinine) than in males (2.62±2.73 µg/gm creatinine). Remarkably, 85% of females and 66% of males exceeded the reference values for urinary cadmium concentration set by the German Human Biomonitoring (HBM) Commission (HBM I and II). Genotype frequencies were 65.4% homozygote typical (CC), 31.0% heterozygote (CA), and 3.6% homozygote atypical (AA). The C allele frequency was 80.9%, while the A allele frequency was 19.1%. Notably, the DMT1 IVS4+44C/A polymorphism significantly influenced urinary cadmium levels, with the CA genotype showing higher levels than CC and AA genotypes. Urinary cadmium levels were also statistically increased with the presence of the A allele (A+ = CA+AA) compared to its absence (A- = CC). Furthermore, our analysis revealed that individuals with the CC genotype more frequently surpass the reference values for urinary cadmium in HBM I and II across all age groups despite their overall urinary cadmium levels not being high.
Conclusion: This study indicates that the CA genotype may signify susceptibility to prolonged cadmium exposure, given its association with elevated urinary cadmium levels. Additional research is essential for a thorough grasp of the implications of DMT1 gene polymorphisms on health outcomes and for establishing monitoring measures for populations residing in cadmium-contaminated areas.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Swaddiwudhipong W, Limpatanachote P, Mahasakpan P, et al. Cadmium-exposed population in Mae Sot District, Tak Province: 1. Prevalence of high urinary cadmium levels in the adults. J Med Assoc Thai. 2007; 90(1): 143-8.
Boonprasert K, Kongjam P, Limpatanachote P, et al. Urinary and blood cadmium levels in relation to types of food and water intake and smoking status in a Thai population residing in cadmium-contaminated areas in Mae Sot. Southeast Asian J Trop Med Public Health. 2011; 42(6): 1521-30.
Pollution Control Department. Cadmium contamination in Mae Tao Creek, Mae Sot District, Tak Province. Bangkok, Thailand: Ministry of Natural Resources and Environment. 2004.
Sriprachote A, Kanyawongha P, Ochiai K, Matoh T. Current situation of cadmium-polluted paddy soil, rice and soybean in the Mae Sot District, Tak Province, Thailand. Soil Sci Plant Nutr. 2012; 58(3): 349-59. doi.org/10.1080/00380768.2012.686435.
La-Up A, Wiwatanadate P, Pruenglampoo S, Uthaikhup S. Recommended rice intake levels based on average daily dose and urinary excretion of cadmium in a cadmium-contaminated area of northwestern Thailand. Toxicol Res. 2017; 33(4): 291-7. doi: 10.5487/TR.2017.33.4.291.
Huff J, Lunn RM, Waalkes MP, Tomatis L, Infante PF. Cadmium-induced cancers in animals and in humans. Int J Occup Environ Health. 2007; 13(2): 202-12. doi: 10.1179/oeh.2007.13.2.202.
Agency for Toxic Substances and Disease Registry. Toxicological profile for Cadmium. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. 2012. Available from: https://wwwn. cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx
Järup L, Rogenfelt A, Elinder CG, et al. Biological half-time of cadmium in the blood of workers after cessation of exposure. Scand J Work Environ Health. 1983; 9(4): 327-31.
doi: 10.1007/s40572- 016-0107-y.
Kayaalti Z, Odabaşi M, Söylemezoğlu T. Genotype and allele frequencies of divalent metal transporter 1 polymorphism in Turkish population. Mol Biol Rep. 2011; 38(4): 2679-84.
doi: 10.1007/s11033-010- 0410-x.
Kayaaltı Z, Akyüzlü DK, Söylemezoğlu T. Evaluation of the effect of divalent metal transporter 1 gene polymorphism on blood iron, lead and cadmium levels. Environ Res. 2015; 137: 8-13.
doi: 10.1016/j. envres.2014.11.008.
Klaassen CD, Liu J, Diwan BA. Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol. 2009; 238(3): 215–20. doi: 10.1016/j. taap.2009.03.026.
Nordberg M, Nordberg GF. Metallothionein and cadmium toxicology-historical review and commentary. Biomolecules. 2022; 12(3). doi: 10.3390/biom1203 0360.
Lane DJR, Merlot AM, Huang ML-H, et al. Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease. Biochim Biophys Acta. 2015; 1853(5): 1130-44. doi: 10.1016/j. bbamcr.2015.01.021.
Thévenod F, Lee W-K, Garrick MD. Iron and cadmium entry into renal mitochondria: physiological and toxicological implications. Front cell Dev Biol. 2020; 8: 848. doi: 10.3389/fcell.2020.00848.
Menon AV, Chang J, Kim J. Mechanisms of divalent metal toxicity in affective disorders. Toxicology. 2016; 339: 58-72. doi: 10.1016/j.tox.2015.11.001.
Tallkvist J, Bowlus CL, Lönnerdal B. DMT1 gene expression and cadmium absorption in human absorptive enterocytes. Toxicol Lett. 2001; 122(2): 171-7. doi: 10.1016/s0378-4274(01)00363-0.
Jakubowski M, Trzcinka-Ochocka M. Biological monitoring of exposure: trends and key developments. J Occup Health. 2005; 47(1): 22-48. doi: 10.1539/joh.47.22.
Sikaphana S, Boonthum R, Leudang S, Parnmen S. Associations between urinary excretion of cadmium with alpha-1 microglobulin and microalbuminuria: a cross-sectional study in northwestern Thai population. J Assoc Med Sci. 2021; 54(2): 35-41. Retrieved from https://he01.tci-thaijo.org/index. php/bulletinAMS/article/view/247385
Chong WS, Kwan PC, Chan LY, et al. Expression of divalent metal transporter 1 (DMT1) isoforms in first trimester human placenta and embryonic tissues. Hum Reprod. 2005; 20(12): 3532-8.
doi: 10.1093/ humrep/dei246.
Ingrassia R, Garavaglia B, Memo M. DMT1 expression and iron levels at the crossroads between aging and neurodegeneration. Front Neurosci. 2019; 13:575. doi: 10.3389/fnins.2019.00575.
He Q, Du T, Yu X, et al. DMT1 polymorphism and risk of Parkinson’s disease. Neurosci Lett. 2011; 501(3): 128-31. doi: 10.1016/j.neulet.2011.07.001.
Garrick MD, Singleton ST, Vargas F, et al. DMT1: which metals does it transport? Biol Res. 2006; 39(1): 79- 85. doi: 10.4067/s0716-97602006000100009.
Peana M, Pelucelli A, Chasapis CT, et al. Biological effects of human exposure to environmental cadmium. Biomolecules. 2022; 13(1): 36. doi: 10.33 90/biom13010036.
Przybyłkowski A, Gromadzka G, Członkowska A. Polymorphisms of metal transporter genes DMT1 and ATP7A in Wilson’s disease. J Trace Elem Med Biol. 2014; 28(1): 8-12.
doi: 10.1016/j.jtemb.2013.08. 002.
Kim H-K, Lee H, Kim H-J. A polymorphism in DMT1 is associated with lead-related hypertensive status. Mol Cell Toxicol. 2013; 9(4): 415-20. doi: 10.1007/ s13273-013-0051-y.
Tolone C, Bellini G, Punzo F, et al. The DMT1 IVS4+44C>A polymorphism and the risk of iron deficiency anemia in children with celiac disease. PLoS One. 2017; 12(10): e0185822.
Wysokinski D, Zaras M, Dorecka M, et al. An association between environmental factors and the IVS4+44C>A polymorphism of the DMT1 gene in agerelated macular degeneration. Graefe’s Arch Clin Exp Ophthalmol. 2012; 250(7): 1057-65. doi: 10.1007/ s00417-012-1966-z.