Renal adverse events of immune checkpoint inhibitors
Main Article Content
Abstract
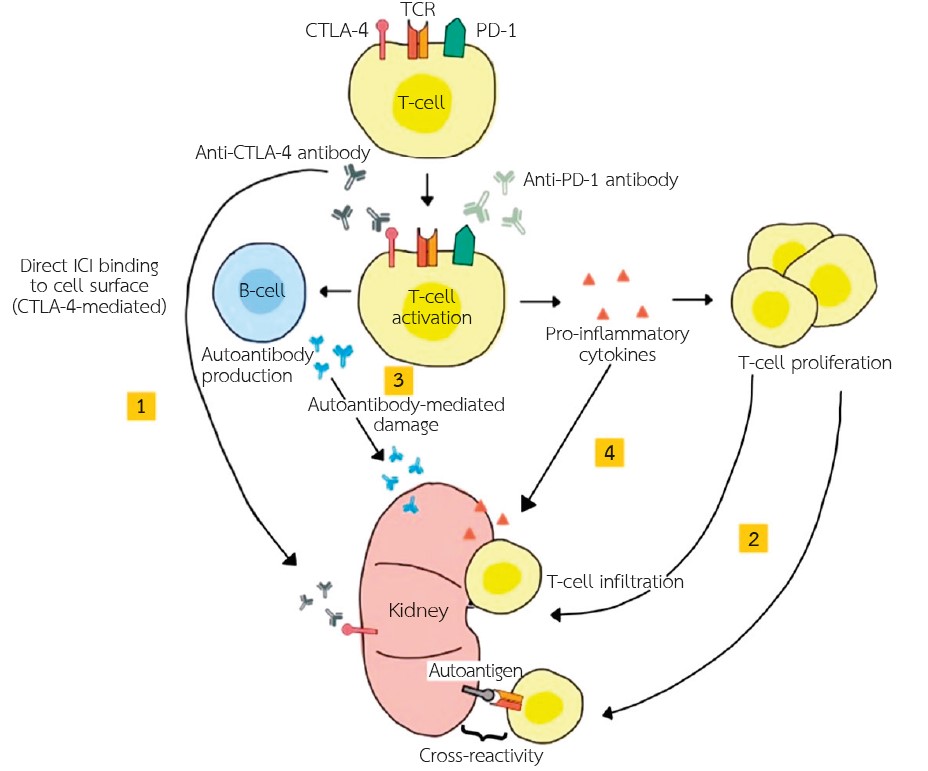
Immune checkpoint inhibitors are new cancer immunotherapies based on the principle of stimulating the immune system to eliminate cancer cells. There are three types of immune checkpoint inhibitors including anti-CTLA4 antibody, anti-PD-1 antibody, and anti-PD-1L antibody. The mechanism of action involves the inhibition of the immune checkpoint, thereby activating T-cells to destroy cancer cells. However, enhancing T-cell activity also leads to immune-related adverse events that can affect virtually any organ. The reported adverse events in the kidneys include electrolyte imbalance, glomerular disease, acute kidney injury and acute rejection in kidney transplants. Acute kidney injury occurs in 2-5% and is presumed to be immune-related resulting in acute tubulointerstitial nephritis. Withholding the drug and administration of corticosteroid are the mainstays of treatment. The medication may be rechallenged after the improvement of renal function. Glomerular disease, although uncommon, can be manifested as nephrotic or nephritic syndrome with variable pathology. The heightened T-cell activation is likely the cause of acute rejection in kidney transplants. There are currently no recommended treatments, therefore, it is advisable to monitor the patient closely for any possible side effects.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This article is published under CC BY-NC-ND 4.0 license, which allows for non-commercial reuse of the published paper as long as the published paper is fully attributed. Anyone can share (copy and redistribute) the material in any medium or format without having to ask permission from the author or the Nephrology Society of Thailand.
References
Leach DR, Krummel MF, Allison JP. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996;271:1734–6.
Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med 2016;375:1767–78.
Halloran PF. Immunosuppressive Drugs for Kidney Transplantation. N Engl J Med 2004;351:2715–29.
Gupta S, Cortazar FB, Riella LV, Leaf DE. Immune Checkpoint Inhibitor Nephrotoxicity: Update 2020. Kidney360 2020;1:130–40.
Shingarev R, Glezerman IG. Kidney Complications of Immune Checkpoint Inhibitors: A Review. Am J Kidney Dis 2019;74:529–37.
Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 2016;27:559–74.
Sprangers B, Leaf DE, Porta C, Soler MJ, Perazella MA. Diagnosis and management of immune checkpoint inhibitorassociated acute kidney injury. Nat Rev Nephrol 2022;18:794–805.
Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378:158–68.
Sury K, Perazella MA, Shirali AC. Cardiorenal complications of immune checkpoint inhibitors. Nat Rev Nephrol 2018;14:571–88.
Uppal NN, Workeneh BT, Rondon-Berrios H, Jhaveri KD. Electrolyte and Acid-Base Disorders Associated with Cancer Immunotherapy. Clin J Am Soc Nephrol 2022;17:922–33.
Seethapathy H, Rusibamayila N, Chute DF, Lee M, Strohbehn I, Zubiri L, et al. Hyponatremia and other electrolyte abnormalities in patients receiving immune checkpoint inhibitors. Nephrol Dial Transplant 2021;36:2241–7.
Manohar S, Kompotiatis P, Thongprayoon C, Cheungpasitporn W, Herrmann J, Herrmann SM. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol Dial Transplant 2019;34:108–17.
Caturegli P, Di Dalmazi G, Lombardi M, Grosso F, Larman HB, Larman T, et al. Hypophysitis Secondary to Cytotoxic T-Lymphocyte–Associated Protein 4 Blockade. Am J Pathol 2016;186:3225–35.
Workeneh BT, Jhaveri KD, Rondon-Berrios H. Hyponatremia in the cancer patient. Kidney Int 2020;98:870–82.
Gupta S, Short SAP, Sise ME, Prosek JM, Madhavan SM, Soler MJ, et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer 2021;9:e003467.
Gérard AO, Andreani M, Fresse A, Parassol N, Muzzone M, Pinel S, et al. Immune checkpoint inhibitors-induced nephropathy: a French national survey. Cancer Immunol Immunother 2021;70:3357–64.
Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer 2019;7:2.
Ashour T, Nakhoul G, Patil P, Funchain P, Herlitz L. Immune Check Point Inhibitor–Associated Glomerulonephritis. Kidney Int Rep 2019;4:355–9.
Jung K, Zeng X, Bilusic M. Nivolumab-associated acute glomerulonephritis: a case report and literature review. BMC Nephrol 2016;17:188.
Fadel F, Karoui KE, Knebelmann B. Anti-CTLA4 Antibody–Induced Lupus Nephritis. N Engl J Med 2009;361:211–2.
Kitchlu A, Jhaveri KD, Wadhwani S, Deshpande P, Harel Z, Kishibe T, et al. A Systematic Review of Immune Checkpoint Inhibitor–Associated Glomerular Disease. Kidney Int Rep 2021;6:66–77.
Perazella MA, Shirali AC. Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do. Kidney Int 2020;97:62–74.
Bermejo S, Bolufer M, Riveiro-Barciela M, Soler MJ. Immunotherapy and the Spectrum of Kidney Disease: Should We Individualize the Treatment. Front Med 2022;9:906565.
Meraz-Muñoz A, Amir E, Ng P, Avila-Casado C, Ragobar C, Chan C, et al. Acute kidney injury associated with immune checkpoint inhibitor therapy: incidence, risk factors and outcomes. J Immunother Cancer 2020;8:e000467.
Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638–47.
Seethapathy H, Zhao S, Chute DF, Zubiri L, Oppong Y, Strohbehn I, et al. The Incidence, Causes, and Risk Factors of Acute Kidney Injury in Patients Receiving Immune Checkpoint Inhibitors. Clin J Am Soc Nephrol 2019;14:1692–700.
Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, et al. Clinical Features and Outcomes of Immune Checkpoint Inhibitor–Associated AKI: A Multicenter Study. J Am Soc Nephrol 2020;31:435–46.
Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. Management of Immunotherapy-Related Toxicities, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Nati Compr Canc Netw 2019;17:255–89.
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–68.
Gupta S, Hanna PE. Immune Checkpoint Inhibitors and the Kidney: An Update. Kidney News 2022;14:22–3.
Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C, et al. Adverse Renal Effects of Immune Checkpoint Inhibitors: A Narrative Review. Am J Nephrol 2017;45:160–9.
Izzedine H, Mathian A, Champiat S, Picard C, Mateus C, Routier E, et al. Renal toxicities associated with pembrolizumab. Clin Kidney J 2019;12:81–8.
Moss EM, Perazella MA. The role of kidney biopsy in immune checkpoint inhibitor nephrotoxicity. Front Med 2022;9:964335.
Harmankaya K, Erasim C, Koelblinger C, Ibrahim R, Hoos A, Pehamberger H, et al. Continuous systemic corticosteroids do not affect the ongoing regression of metastatic melanoma for more than two years following ipilimumab therapy. Med Oncol 2011;28:1140–4.
Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol 2017;35:785–92.
Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59–67.
US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2016;67:A4.
Sprangers B, Nair V, Launay-Vacher V, Riella LV, Jhaveri KD. Risk factors associated with post–kidney transplant malignancies: an article from the Cancer-Kidney International Network. Clin Kidney J 2018;11:315–29.
Murakami N, Mulvaney P, Danesh M, Abudayyeh A, Diab A, Abdel-Wahab N, et al. A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int 2021;100:196–205.
Venkatachalam K, Malone AF, Heady B, Santos RD, Alhamad T. Poor Outcomes With the Use of Checkpoint Inhibitors in Kidney Transplant Recipients. Transplantation 2020;104:1041–7.
Izzedine H, Gueutin V. Toxicités rénales des inhibiteurs des points de contrôle de l’auto-immunité. Nephrol Ther 2020;16:19–26.
The 3C Study Collaborative Group, Haynes R, Blackwell L, Staplin N, Herrington WG, Emberson J, et al. Campath, calcineurin inhibitor reduction, and chronic allograft nephropathy (the 3C Study) – results of a randomized controlled clinical trial. Am J Transplant 2018;18:1424–34.
Daanen RA, Maas RJH, Koornstra RHT, Steenbergen EricJ, van Herpen CML, Willemsen AECAB. Nivolumab-associated Nephrotic Syndrome in a Patient With Renal Cell Carcinoma: A Case Report. J Immunother 2017;40:345–8.
Kitchlu A, Fingrut W, Avila-Casado C, Chan CT, Crump M, Hogg D, et al. Nephrotic Syndrome With Cancer Immunotherapies: A Report of 2 Cases. Am J Kidney Dis 2017;70:581–5.