A comparative study of diagnostic performance for tuberculosis and rifampicin- resistant tuberculosis between standard and pooled sputum methods using GeneXpert Ultra at Lampang Hospital, Thailand
Main Article Content
Abstract
Background: Molecular diagnosis using the Xpert MTB/RIF Ultra assay enables rapid detection of Mycobacterium tuberculosis and rifampicin resistance.
Objectives: This study evaluated the diagnostic performance and cost efficiency of a two-sample pooled testing approach compared with standard individual testing.
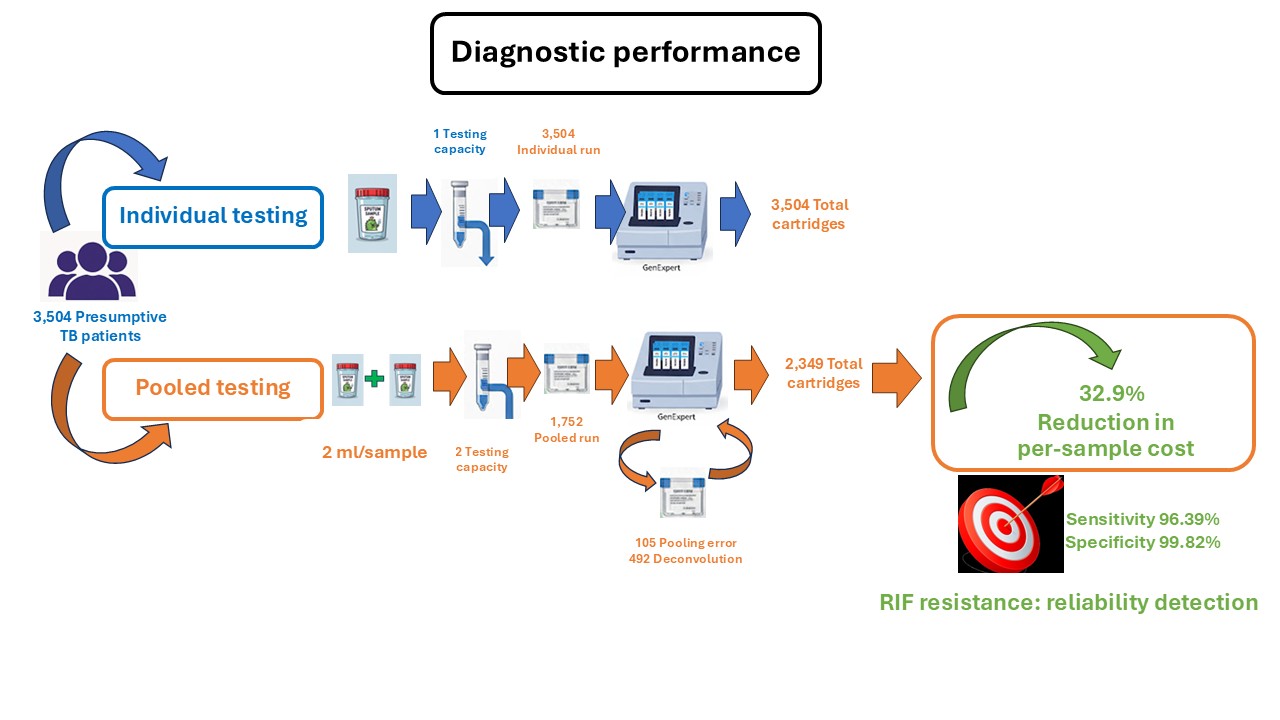
Materials and methods: A cross-sectional diagnostic study was conducted among 3,504 presumptive TB patients. Sputum specimens were tested by Xpert Ultra individually, with those results serving as the reference standard, and in two-sample pools. The pooling protocol utilized 2.0 mL from each specimen to maximize load volume. Deconvolution, which required retesting both individual specimens, was mandatory for all positive and invalid pooled results. Performance metrics, RIF resistance concordance, and cost-effectiveness modeled on cartridge consumption and direct cost 550 Thai Baht per unit were compared.
Results: The pooled method showed excellent concordance with the individual method, yielding 240 concordant positives and 3,249 concordant negatives, with no statistically significant difference in MTB detection (McNemar χ²=0.267, p=0.605). The pooled approach achieved a sensitivity of 96.39% (95% CI, 93.25-98.33) and a specificity of 99.82% (95% CI, 99.60-99.93). The assay maintained reliable detection of RIF resistance, indicating that pooling did not compromise molecular accuracy. In practice, 1,752 pooled runs were performed, with 105 (5.7%) error results and 246 positive pools requiring deconvolution. Including repeats and deconvolution, total cartridge use was 2,349 compared with 3,504 for individual testing, corresponding to an actual cost reduction of 32.96% (approx. 180,000 Thai Baht saved per 1,000 tests).
Conclusion: Two-sample pooled Xpert Ultra testing demonstrated high diagnostic accuracy and doubled analytical throughput. Although deconvolution limited cost savings to approximately 33%, the strategy proved highly cost-effective and operationally feasible. This method offers a practical, scalable approach for optimizing molecular TB diagnostics and resource utilization, especially in low- to moderate-prevalence settings.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
WHO consolidated guidelines on tuberculosis. Module 3: diagnosis–rapid diagnostics for tuberculosis detection: World Health Organization; 2024. Available from: https://www.who.int/publications/i/item/9789240089488.
Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nat Rev Dis Primers. 2016; 2(1): 16076. doi: 10.1038/nrdp.2016.76.
Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database of Sys Rev. 2014; 2014(1): CD009593. doi: 10.1002/14651858.CD009593.pub3.
Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010; 363(11): 1005-15. doi: 10.1056/NEJMoa0907847.
Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018; 18(1): 76-84. doi: 10.1016/S1473-3099(17)30691-6.
Abdalhamid B, Bilder CR, Garrett JL, Iwen PC. Cost effectiveness of sample pooling to test for SARS-CoV-2. Journal of infection in developing countries. 2020; 14(10): 1136. doi: doi.org/10.3855/jidc.13935.
Iem V, Chittamany P, Suthepmany S, Siphanthong S, Somphavong S, Kontogianni K, et al. Pooling sputum for Xpert MTB/RIF and Xpert Ultra testing during the Covid-19 pandemic in Lao People’s Democratic Republic. PLOS global public health. 2022; 2(4): e0000116. doi: 10.1371/journal.pgph.0000116.
Zifodya JS, Kreniske JS, Schiller I, Kohli M, Dendukuri N, Schumacher SG, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst Rev. 2021; 2(2): Cd009593. doi: 10.1002/14651858.CD009593.pub5.
Vuchas C, Teyim P, Dang BF, Neh A, Keugni L, Che M, et al. Implementation of large-scale pooled testing to increase rapid molecular diagnostic test coverage for tuberculosis: a retrospective evaluation. Sci Rep. 2023; 13(1): 15358. doi: 10.1038/s41598-023-41904-w.
Iem V, Bimba JS, Santos VS, Dominguez J, Creswell J, Somphavong S, et al. Pooling sputum testing to diagnose tuberculosis using xpert MTB/RIF and xpert ultra: a cost-effectiveness analysis. BMC Infect Dis. 2023; 23(1): 341. doi: 10.1186/s12879-023-08330-9.
Iem V, Xangsayarath P, Chittamany P, Suthepmany S, Siphanthong S, Paboriboune P, et al. Pooling samples to increase testing capacity with Xpert Xpress SARS-CoV-2 during the Covid-19 pandemic in Lao People’s Democratic Republic. PLoS One. 2022; 17(9): e0275294. doi: 10.1371/journal.pone.0275294.
Zeng J, Huang H, Liu X, Huang Z, Liu W, Liu H, et al. Pooling sputum samples for the Xpert MTB/RIF assay: a practical screening strategy for highly infectious tuberculosis cases. BMC Infectious Diseases. 2024; 24(1): 122. doi: 10.1186/s12879-024-09020-w.
Bimba J, Adekeye O, Iem V, Eliya T, Osagie I, Kontogianni K, et al. Pooling sputum samples for Xpert® MTB/RIF and Xpert® Ultra testing for TB diagnosis. Public Health Action. 2023; 13(1): 12-6. doi: 10.5588/pha.22.0052.
World Health Organization consolidated guidelines on tuberculosis. Module 3: Diagnosis: World Health Organization; 2025. Available from: https://www.who.int/publications/i/item/9789240107984.
Bogere N, Bongomin F, Katende A, Ssebambulidde K, Ssengooba W, Ssenfuka H, et al. Performance and cost-effectiveness of a pooled testing strategy for SARS-CoV-2 using real-time polymerase chain reaction in Uganda. International Journal of Infectious Diseases. 2021; 113: 355-8. doi: 10.1016/j.ijid.2021.10.038.
Yelin I, Aharony N, Tamar ES, Argoetti A, Messer E, Berenbaum D, et al. Evaluation of COVID-19 RT-qPCR Test in Multi sample Pools. Clinical Infectious Diseases. 2020; 71(16): 2073-8. doi: 10.1093/cid/ciaa531.