Icaritin delivered by hyaluronic acid-modified liposome enhanced apoptosis and anti-metastasis of Huh7 liver cancer cells
Main Article Content
Abstract
Objectives: The advancement of tumor-targeted drug delivery systems, particularly those utilizing hyaluronic acid (HA)-modified nanocarriers, presents a promising strategy for enhancing cancer therapy by specifically targeting CD44-overexpressing tumor cells. In this study, HA-functionalized liposomes (HA-Lip) were employed to deliver the anti-cancer drug icaritin (ICT) to liver cancer cells to overcome its poor water solubility and low bioactivity.
Materials and methods: The efficacy of HA-Lip-ICT was evaluated through a combination of in vitro assays, including reactive oxygen species (ROS) production measurement, cell migration and invasion assays using Huh7 liver cancer cells. RNA sequencing and Western blot analysis were used to elucidate the molecular mechanisms underlying ICT’s anti-cancer effects.
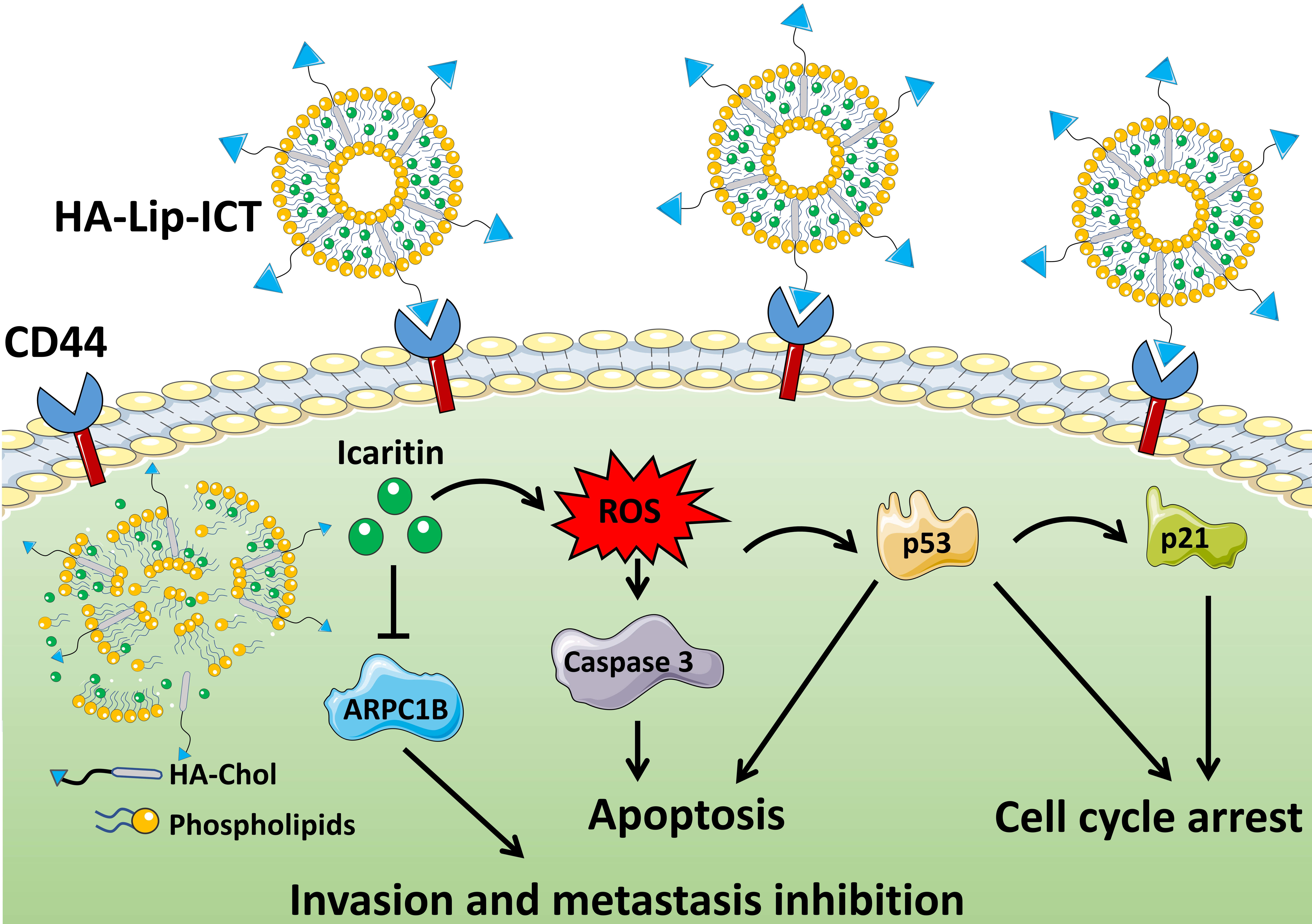
Results: HA-Lip-ICT significantly increased the production of reactive oxygen species (ROS) induced by icaritin and effectively inhibited the migration and invasion of Huh7 liver cancer cells in vitro through HA/CD44 interactions. RNA sequencing and Western blot analysis revealed that icaritin inhibited liver cancer progression by promoting apoptosis, inducing cell cycle arrest, and reducing metastasis. Notably, HA-Lip-ICT demonstrated a tumor migration inhibition rate of 55.6% and down-regulated the metastasis closely related protein ARPC1B. Moreover, it significantly upregulated proteins associated with apoptosis and cell cycle regulation, including caspase 3a, p53, and p21.
Conclusion: The research highlights that HA-Lip-ICT presents considerable promise as a targeted drug delivery system for tumors, particularly in enhancing liver cancer treatment by reducing tumor spread and facilitating cell death
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65: 87-108. doi: 10.3322/caac.21262.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66: 115-32. doi: 10.3322/caac.21338.
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007; 132: 2557-76. doi: 10.1053/j.gastro.2007.04.061.
Jing P, Luo H, Tan J, Liao C, Zhang S. Natural polyphenol-loaded cross-linked lipoic acid vesicles treat triple-negative breast cancer by cancer cell killing and metastasis inhibition. Mater Des. 2023; 236: 112461. doi: 10.1016/j.matdes.2023.112461.
Tan J, Jing P, Xiao X, Liao Y, Liao C, Zhang S. Cross-linked lipoic acid nanocapsules serve as H2O2 amplifier to strengthen the H2O2-sensitive prodrug activation. Sci China Chem. 2023; 66: 2654-63. doi: 10.1007/s11426-022-1647-2.
Singh S, Singh PP, Roberts LR, Sanchez W. Chemo-preventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2014; 11: 45-54. doi: 10.1038/nrgastro.2013.143.
Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005; 100: 72-9. doi: 10.1016/j.jep.2005.05.011.
Newman DJ. Natural products as leads to potential drugs: an old process or the new hope for drug discovery? J Med Chem. 2008; 51: 2589-99. doi: 10.1021/jm0704090.
Sze SC, Tong Y, Ng TB, Cheng CL, Cheung HP. Herba Epimedii: anti-oxidative properties and its medical implications. Molecules. 2010; 15: 7861-70. doi: 10.3390/molecules15117861.
Bailly C. Molecular and cellular basis of the anticancer activity of the prenylated flavonoid icaritin in hepatocellular carcinoma. Chem Biol Interact. 2020; 325: 109124. doi: 10.1016/j.cbi.2020.109124.
Li Y, Sun S, Chang Q, Zhang L, Wang G, Chen W, et al. A strategy for the improvement of the bioavailability and antiosteoporosis activity of BCS IV flavonoid glycosides through the formulation of their lipophilic aglycone into nanocrystals. Mol Pharm. 2013; 10: 2534-42. doi: 10.1021/mp300688t.
Chang Q, Wang GN, Li Y, Zhang L, You C, Zheng Y. Oral absorption and excretion of icaritin, an aglycone and also active metabolite of prenylflavonoids from the Chinese medicine Herba Epimedii in rats. Phytomedicine. 2012; 19: 1024-8. doi: 10.1016/j.phymed.2012.05.017.
Sabit H, Abdel-Hakeem M, Shoala T, Abdel-Ghany S, Abdel-Latif MM, Almulhim J, et al. Nanocarriers: A reliable tool for the delivery of anticancer drugs. Pharmaceutics. 2022; 14(8): 1566. doi: 10.3390/pharmaceutics14081566.
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021; 20: 101-24. doi: 10.1038/s41573-020-0090-8.
Lee MK. Liposomes for enhanced bioavailability of water-insoluble drugs: In Vivo evidence and recent approaches. Pharmaceutics. 2020; 12(3): 264. doi: 10.3390/pharmaceutics12030264.
Cheng R, Liu L, Xiang Y, Lu Y, Deng L, Zhang H, et al. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials. 2020; 232: 119706. doi: 10.1016/j.biomaterials.2019.119706.
Kesharwani P, Chadar R, Sheikh A, Rizg WY, Safhi AY. CD44-targeted nanocarrier for cancer therapy. Front Pharmacol. 2021; 12: 800481. doi: 10.3389/fphar.2021.800481.
Su S, Kang P. Recent advances in nanocarrier-assisted therapeutics delivery systems. Pharmaceutics. 2020; 12(9): 837. doi: 10.3390/pharmaceutics12090837.
Majumder J, Taratula O, Minko T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv Drug Deliv Rev. 2019; 144: 57-77. doi: 10.1016/j.addr.2019.07.010.
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018; 16: 71. doi: 10.1186/s12951-018-0392-8.
Endo K, Terada T. Protein expression of CD44 (standard and variant isoforms) in hepatocellular carcinoma: relationships with tumor grade, clinicopathologic parameters, p53 expression, and patient survival. J Hepatol. 2000; 32: 78-84. doi: 10.1016/s0168-8278(00)80192-0.
Asai R, Tsuchiya H, Amisaki M, Makimoto K, Takenaga A, Sakabe T, et al. CD44 standard isoform is involved in maintenance of cancer stem cells of a hepatocellular carcinoma cell line. Cancer Med. 2019; 8: 773-82. doi: 10.1002/cam4.1968.
Cannito S, Bincoletto V, Turato C, Pontisso P, Scupoli MT, Ailuno G, et al. Hyaluronated and PEGylated liposomes as a potential drug-delivery strategy to specifically target liver cancer and inflammatory cells. Molecules. 2022; 27(3): 1062. doi: 10.3390/molecules
Sun X, He Z, Lu R, Liu Z, Chiampanichayakul S, Anuchapreeda S, et al. Hyaluronic acid-modified liposomes Potentiated in-vivo anti-hepatocellular carcinoma of icaritin. Front Pharmacol. 2024; 15: 1437515. doi: 10.3389/fphar.2024.1437515.
Ohvo-Rekila H, Ramstedt B, Leppimaki P, Slotte JP. Cholesterol interactions with phospholipids in membranes. Prog Lipid Res. 2002; 41: 66-97. doi: 10.1016/s0163-7827(01)00020-0.
Song M, Liang Y, Li K, Zhang J, Zhang N, Tian B, et al. Hyaluronic acid modified liposomes for targeted delivery of doxorubicin and paclitaxel to CD44 over-expressing tumor cells with improved dual-drugs synergistic effect. J Drug Deliv Sci Technol. 2019; 53: 101179. doi: 10.1016/j.jddst.2019.101179.
Wang S, Wang Q, Wang H, Qin C, Cui X, Li L, et al. Induction of ROS and DNA damage-dependent senescence by icaritin contributes to its antitumor activity in hepatocellular carcinoma cells. Pharm Biol. 2019; 57: 424-31. doi: 10.1080/13880209.2019.1628073.
Ren F, Li J, Yuan X, Wang Y, Wu K, Kang L, et al. Dandelion polysaccharides exert anticancer effect on Hepatocellular carcinoma by inhibiting PI3K/AKT/mTOR pathway and enhancing immune response. J Funct Foods. 2019; 55: 263-74. doi: 10.1016/j.jff.2019.02.034.
Munakarmi S, Chand L, Shin HB, Hussein UK, Yun BS, Park HR, et al. Anticancer effects of Poncirus fructus on hepatocellular carcinoma through regulation of apoptosis, migration, and invasion. Oncol Rep. 2020; 44: 2537-46. doi: 10.3892/or.2020.7790.
Siqueira EDS, Concato VM, Tomiotto-Pellissier F, Silva TF, Bortoleti B, Goncalves MD, et al. Trans-chalcone induces death by autophagy mediated by p53 up-regulation and beta-catenin down-regulation on human hepatocellular carcinoma HuH7.5 cell line. Phytomedicine. 2021; 80: 153373. doi: 10.1016/j.phymed.2020.153373.
Liu D, Zhang Q, Wang J, Guan S, Cai D, Liu J. Inhibition of growth and metastasis of breast cancer by targeted delivery of 17-hydroxy-jolkinolide B via hyaluronic acid-coated liposomes. Carbohydr Polym. 2021; 257: 117572. doi: 10.1016/j.carbpol.2020.117572.
Wang J, Liu D, Guan S, Zhu W, Fan L, Zhang Q, et al. Hyaluronic acid-modified liposomal honokiol nanocarrier: Enhance antimetastasis and antitumor efficacy against breast cancer. Carbohydr Polym. 2020; 235: 115981. doi: 10.1016/j.carbpol.2020.115981.
Hu X, Ding J, Wang G, Zhang X. The combination of ulinastatin and 5-fluorouracil synergistically inhibits hepatocellular carcinoma growth. J Int Med Res. 2020; 48(3): 0300060520909776. doi: 10.1177/0300060520909776.
Ghafouri-Fard S, Abak A, Tondro Anamag F, Shoorei H, Fattahi F, Javadinia SA, et al. 5-Fluorouracil: A narrative review on the role of regulatory mechanisms in driving resistance to this chemotherapeutic agent. Front Oncol. 2021; 11: 658636. doi: 10.3389/fonc.2021.
Tran TH, Choi JY, Ramasamy T, Truong DH, Nguyen CN, Choi HG, et al. Hyaluronic acid-coated solid lipid nanoparticles for targeted delivery of vorinostat to CD44 overexpressing cancer cells. Carbohydr Polym. 2014; 114: 407-15. doi: 10.1016/j.carbpol.2014.08.026.
Jeong SH. Inhibitory effect of phytol on cellular senescence. Biomed Dermatol. 2018; 2: 1-9. doi: 10. 1186/s41702-018-0025-8.
Yu Z, Guo J, Hu M, Gao Y, Huang L. Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma. ACS Nano. 2020; 14: 4816-28. doi: 10.1021/acsnano.0c00708.
Li H, Liu Y, Jiang W, Xue J, Cheng Y, Wang J, et al. Icaritin promotes apoptosis and inhibits proliferation by down-regulating AFP gene expression in hepatocellular carcinoma. BMC Cancer. 2021; 21: 318. doi: 10.1186/s12885-021-08043-9.
Gao L, Ouyang Y, Li R, Zhang X, Gao X, Lin S, et al. Icaritin inhibits migration and invasion of human ovarian cancer cells via the Akt/mTOR signaling pathway. Front Oncol. 2022; 12: 843489. doi: 10.3389/fonc.2022.843489.
Li X, Li C, Zhou P, Chen S. Inhibitory effect of icaritin on proliferation, migration, and invasion of human nasopharyngeal carcinoma cell CNE2 by regulating STAT3 activation. Pharmazie. 2019; 74: 685-7. doi: 10.1691/ph.2019.9632.
Xiao Y, Yao W, Lin M, Huang W, Li B, Peng B, et al. Icaritin-loaded PLGA nanoparticles activate immunogenic cell death and facilitate tumor recruitment in mice with gastric cancer. Drug Deliv. 2022; 29: 1712-25. doi: 10.1080/10717544.2022.2079769.
Huang J, Zhou H, Tan C, Mo S, Liu T, Kuang Y. The overexpression of actin related protein 2/3 complex subunit 1B(ARPC1B) promotes the ovarian cancer progression via activation of the Wnt/beta-catenin signaling pathway. Front Immunol. 2023; 14: 1182677. doi: 10.3389/fimmu.2023.1182677.
Gamallat Y, Zaaluk H, Kish EK, Abdelsalam R, Liosis K, Ghosh S, et al. ARPC1B is associated with lethal prostate cancer and its inhibition decreases cell invasion and migration In vitro. Int J Mol Sci. 2022; 23: 1476. doi: 10.3390/ijms23031476.
Liu T, Zhu C, Chen X, Wu J, Guan G, Zou C, et al. Dual role of ARPC1B in regulating the network between tumor-associated macrophages and tumor cells in glioblastoma. Oncoimmunology. 2022; 11: 2031499. doi: 10.1080/2162402X.2022.2031499.
Chang D, Du H, Chen X, Bian X, Tian W, Shen J, et al. A controlled random gene perturbation method identifies ARPC1B gene as a key regulator of cancer metastasis. Genes Dis. 2023; 10: 687-9. doi: 10.1016/j.gendis.2022.06.006.
Liao C, Chen W, Xu G, Wang J, Dong W. High expression of ARPC1B correlates with immune infiltration and poor outcomes in glioblastoma. Biochem Biophys Rep. 2024; 37: 101619. doi: 10.1016/j.bbrep.2023.101619.