Analytical errors in the laboratory of a general hospital of the Thai Red Cross Society: A 3-year experience
Main Article Content
Abstract
Background:Laboratory test results are critical to clinical decision-making, including patient screening, diagnosis, treatment planning, and monitoring therapeutic responses. Therefore, laboratory errors can significantly impact patient care and outcomes.
Objectives:To identify and quantify the types and frequencies of errors in the total testing process of the medical laboratory department, with the goal of reducing preventable errors and improving overall quality.
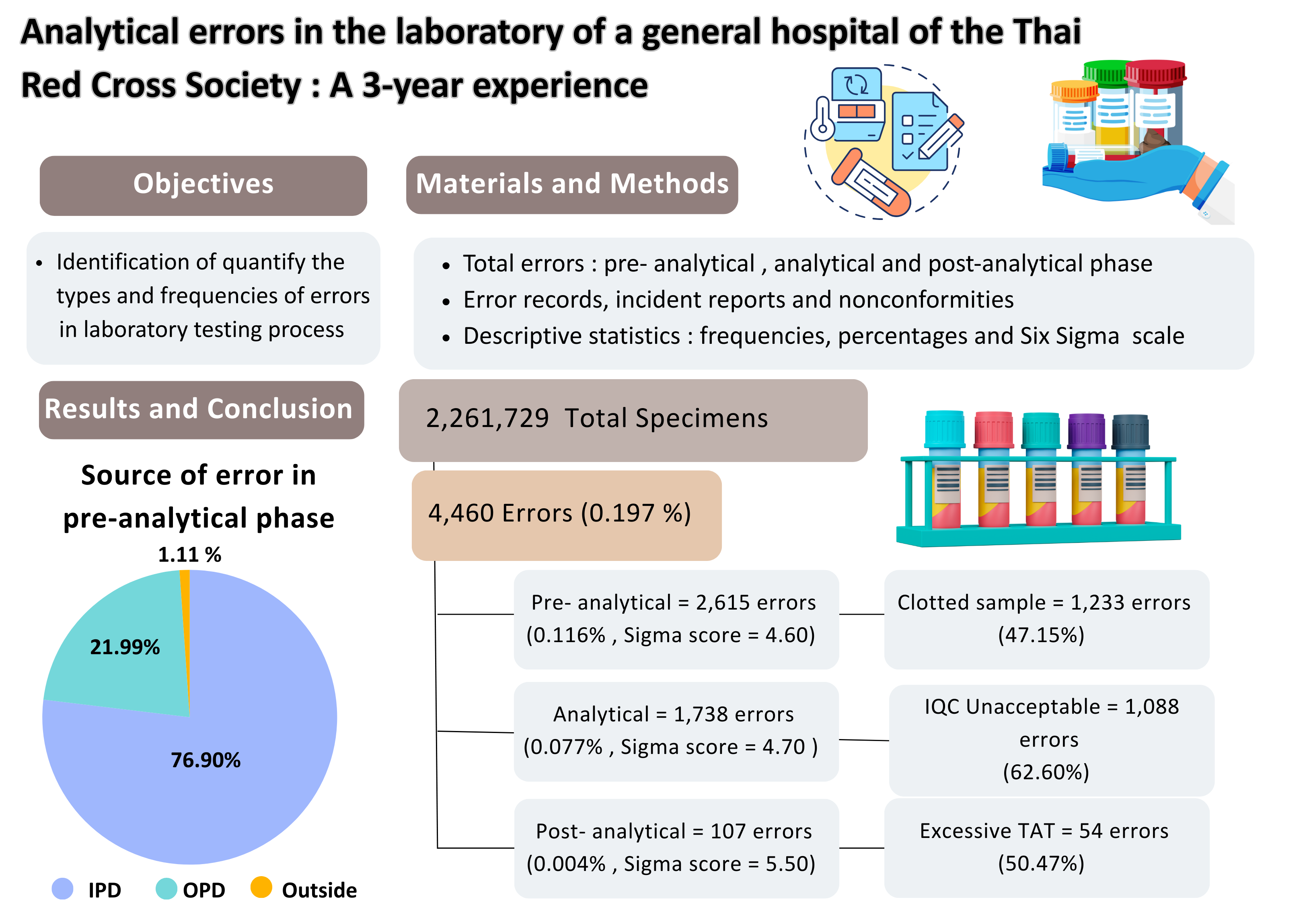
Materials and Methods:This retrospective descriptive study analyzed error records, incident reports and nonconformities from January 2021, to December, 2023. Errors were categorized by testing phase: pre-analytical, analytical and post-analytical. Specimen sources were classified as outpatient, inpatient, or external (outside hospital). Data were summarized using descriptive statistics, including frequencies, percentages and Six Sigma performance scale values.
Results:A total of 2,261,729 specimens were received during the study period. The overall error rate was 0.197% (4,460 errors). Error rates by phase were 0.116% for pre-analytical, 0.077% for analytical, and 0.004% for postanalytical. Six Sigma performance scores were 4.60 (pre-analytical), 4.70 (analytical), and 5.50 (post-analytical), all within acceptable quality thresholds (>4.15, >3.85 and >4.80, respectively). The most common pre-analytical error was clotted samples (1,233 cases). Analytical errors were dominated by unacceptable internal quality control results (1,088 cases). The most frequent post-analytical error was excessive turnaround time (54 cases). Pre-analytical errors occurred most frequently in inpatient specimens (76.90%), with clotted samples accounting for 41.49% of those cases. Six Sigma values by specimen source were 4.20 for inpatients, 4.90 for outpatients and 4.90 for external sources.
Conclusion: Most laboratory errors occurred in the pre-analytical phase, primarily due to specimen collection issues related to quality and volume, particularly in the inpatient setting. Targeted preventative measures-especially in pre-analytical processes-are essential to minimize errors and improve patient safety. Analytical errors were primarily due to unacceptable IQC, EQA. For quality control improvement, training program laboratory staff on quality control, sigma metrics, risk assessment and quality goal index (QGI), including selection and application of Westgard’s rules, is important. The integration of sigma metrics with QGI, risk management and systematic quality control can enhance laboratory performance and reliability of analytical results.enhance laboratory performance and reliability of analytical results.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Vongsakulyanon A. Prevention of clinical laboratory test error: Pre-analytical error. Rama Med J. 2019; 42(2): 49-62. doi: 10.33165/rmj.2019.42.2.142535. [in Thai].
Plebani M. The detection and prevention of errors in laboratory medicine. Ann Clin Biochem. 2010; 47(2): 101-10. doi: 10.1258/acb.2009.009222.
Hammerling JA. A review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012; 43(2): 41-44.
Pratoomtone P. Types and frequency of laboratory errors in Sawanpracharak Hospital, Nakornsawan province: One year after laboratory accreditation. Bull Chiang Mai Assoc Med Sci. 2010; 13: 70-76. [in Thai].
Atay A, Demir L, Cuhadar S, Saglam G, Unal H, Aksum S, et al. Clinical biochemistry laboratory rejection rates due to various types of preanalytical errors. Biochem Med (Zagreb). 2014; 24: 376-82.
Giavarina D, Lippi G. Blood venous sample collection: Recommendations overview and a checklist to improve quality. Clin Biochem. 2017; 50(10-11): 568-73. doi: 10.1016/j.clinbio chem.2017.02.021.
Sanford KW, McPherson RA. Preanalysis. In: McPherson RA, Pincus MR, Editors. Henry’s clinical diagnosis and management by laboratory methods. Philadelphia: Elsevier; 2011: 24-36.
Adcock DM, Favaloro EJ, Lippi G. Critical pre-examination variables in the hemostasis laboratory and their quality indicators. Clin Biochem. 2016; 49(18): 1315-20. doi: 10.1016/j.clinbiochem.2016.08.022.
Comes M, van Dongen-Lases E, Grankvist K, Ibarz M, Kristensen G, Lippi G, et al. Order of blood draw: Opinion paper by the European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working Group for the Preanalytical Phase (WG-PRE). Clin Chem Lab Med. 2017; 55(1): 27-31. doi: 10.1515/cclm-2016-0426.
Cornes M. Case report of unexplained hypocalcaemia in a slightly haemolysed sample. Biochem Med (Zagreb). 2017; 27: 426-9. doi.org/10.11613/BM.2017.046.
Mrazek C, Lippi G, Keppel MH, Felder TK, Oberkofler H, Haschke-Becher E, et al. Errors within the total laboratory testing process, from test selection to medical decision-making: a review of causes, consequences, surveillance and solutions. Biochem Med (Zagreb). 2020; 30(2): 020502: 1-19. doi: 10.11613/BM.2020.020502.
World Health Organization. WHO guidelines on drawing blood: best practices in phlebotomy [Internet]. Geneva: World Health Organization; 2010 [cited 2024Oct30]. Availablefrom: https://iris.who.int/bitstream/handle/10665/44294/9789241599221_eng.pdf?sequence=1.
Westgard JO. Six sigma Calculators. [Internet]. 2009 [cited 2024 Oct 24] Available from:https://www.westgard.com/six-sigma-calculators.htm.
Kimengech KK, Waithaka SK, Onyuka J, Kigondu CS. Determination of errors that compromise the quality of laboratory service in a tertiary hospital. Asian J Med Sci [Internet]. 2017 [cited 2024 Oct 27]; Available from: https://www.pdfs.semanticscholar.org/76C7/ 1b40af4649bf31c11222f439fca02ee429a5.pdf.
Abdollahi A, Saffar Hi, Saffar Ha. Types and frequency of errors during different phase of testing at a clinical medical laboratory of a teaching hospital in Tehran, Iran. North Am J Med Sci [Internet]. 2014; 6: 224-8 [cited : 2024 OCT 28] Available from https://pmc.ncbi.mlm.nih.gov/articles/PMC4049056.
Tola EK, Dabi YT, Dano GT. Assessment of types and frequency of errors in diagnostic laboratories among selected hospitals in East Wollega Zone, Oromia, Ethiopia. Pathol Lab Med Int [Internet]. 2022; 14: 1-6 [cited 2024 Oct 28]. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC4049056.
Zaidi SSH, Sana M, Ghafoor MT. Assessment of total laboratory errors in clinical chemistry laboratory: experience at a tertiary care hospital. Liaquat Natl J Prim Care [Internet]. 2022; 4(1): 6-10 [cited 2024 Oct 28]. Available from: https://journals.lnh.deu.pk/Injpc/pdfl9da637ba-75bd-45bd-8029-6db074353181.pdf.
Choosongsang P, Wannapong N, Choosongsang P, Wilairat B, Treerut P, Musigavon P, et al. Evaluation of analytical errors in the clinical chemistry laboratory of Songklanagarind hospital: A 5-year experience. J Med Tech Phy Ther. 2017; 29(1): 25-33. [in Thai].
Arechep N, Wongkrajang P, Sutasanasuang J, Laiwejpithaya S. Error rate of pre-analytical process at laboratory in Thailand medical school. J Med Tech Assoc Thai. 2024; 52(1): 8933-43. [in Thai].