Evaluation of ciprofloxacin, prednisolone, and infliximab effects on liver functions in acetic acid-induced ulcerative colitis rat model
Main Article Content
Abstract
Background: Ulcerative colitis (UC) is a chronic inflammatory bowel disease characterized by recurring episodes of gastrointestinal inflammation and damage, affecting millions worldwide. Emerging evidence suggests a complex relationship between UC and liver dysfunction, with increased risk of hepatotoxicity and liver-related disorders.
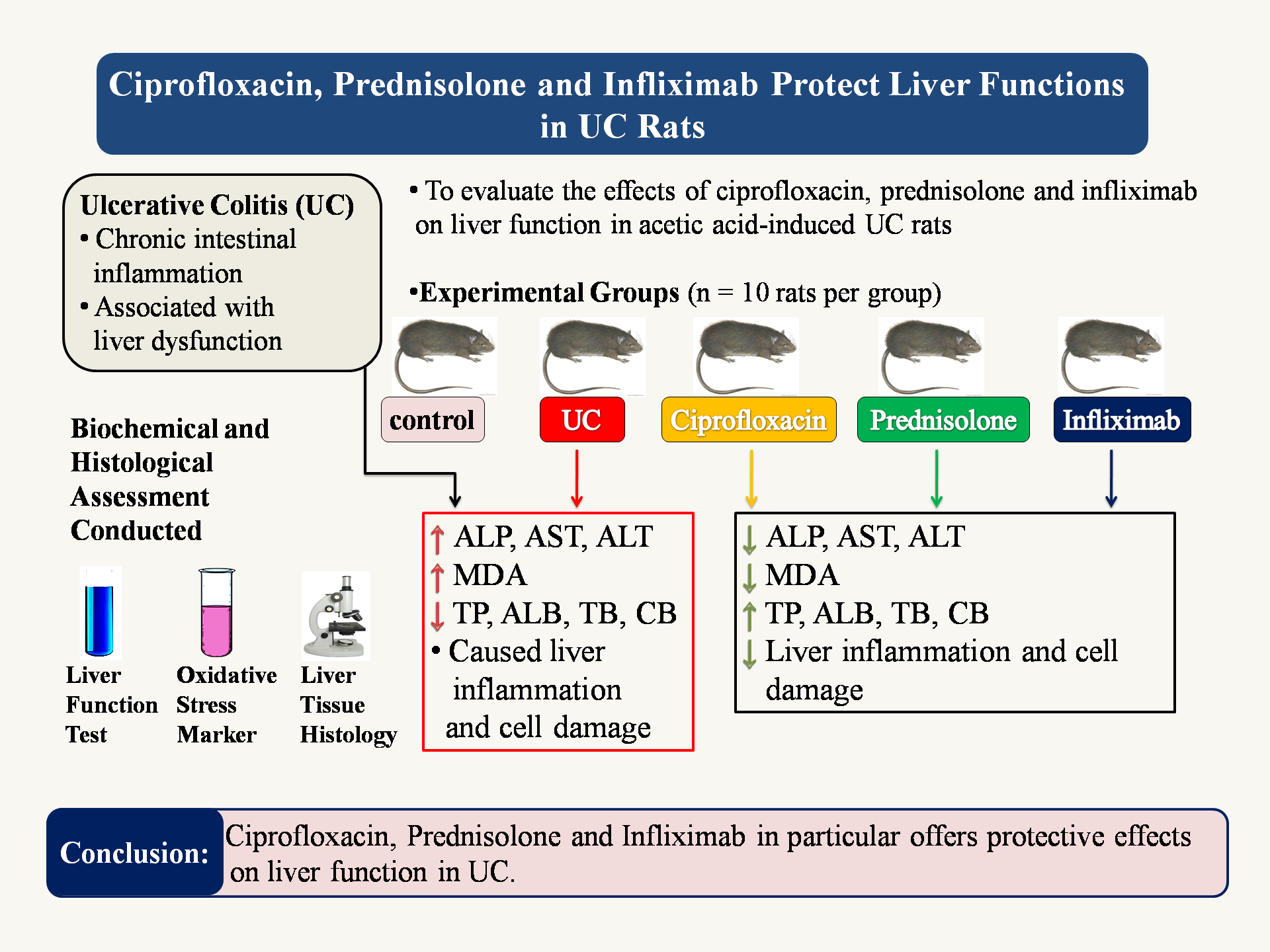
Objectives: To investigate the effects of ciprofloxacin, prednisolone, and infliximab on liver function in an acetic acid-induced UC rat model.
Materials and methods: Fifty male Sprague-Dawley rats were divided into five groups of ten rats each: control, UC, ciprofloxacin, prednisolone, and infliximab group. UC was induced with 2 ml of 4% acetic acid solution transrectal. Liver function tests (AST, ALT, ALP), oxidative stress markers (MDA), protein synthesis (total protein, albumin), and bilirubin processing were evaluated, and histological examination of liver tissues was performed.
Results: The UC group showed significant increase in liver function enzymes (ALP, AST, ALT), oxidative stress markers (MDA) and significant decrease in total protein, albumin, total bilirubin and conjugated bilirubin level when compared with the control group. Respective treatment groups demonstrated improved liver enzyme levels, reduced oxidative stress, enhanced protein synthesis and bilirubin processing. Histological examination revealed improved liver architecture and reduced inflammation in treatment groups when compared with the UC group.
Conclusion: This study provides evidence that ciprofloxacin, prednisolone, and infliximab exert protective effects on liver function in acetic acid induced UC model. These findings suggest potential benefits of these therapies in mitigating liver damage associated with UC, highlighting the importance of considering liver health in UC management.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Gajendran, M., Loganathan, P., Jimenez, G., Catinella, A. P., Ng, N., Umapathy, C., et al. A comprehensive review and update on ulcerative colitis. Dis Mon. 2019; 65(12): 100851. doi:10.1016/j.disamonth. 2019.02.004.
Kucharzik T, Koletzko S, Kannengiesser K, Dignass A. Ulcerative colitis-diagnostic and therapeutic algorithms. DtschArztebl Int. 2020; 117(33-34): 564-74. doi:10.3238/arztebl.2020.0564.
Wieser V, Gerner R, Moschen AR, Tilg H. Liver complications in inflammatory bowel diseases. Dig Dis. 2013; 31(2): 233-8. doi:10.1159/000353377.
Schröder, T., Schmidt, K. J., Olsen, V., Möller, S., Mackenroth, T., Sina, C., et al. Liver steatosis is a risk factor for hepatotoxicity in patients with inflammatory bowel disease under immunosuppressive treatment. Eur J Gastroenterol Hepatol. 2015; 27(6): 698-704. doi:10.1097/MEG.0000000000000350.
Chen M, Suzuki A, Borlak J, Andrade RJ, Lucena MI. Drug-induced liver injury: Interactions between drug properties and host factors. J Hepatol. 2015; 63(2): 503-14. doi:10.1016/j.jhep.2015.04.016.
Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016; 13(3): 267-76. doi:10.1038/cmi.2016.3.
Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory infliximab and opportunities for combination. Clin Cancer Res. 2013; 19(5): 997-1008. doi:10. 1158/1078-0432.CCR-12-2214.
Devarbhavi H, Andrade RJ. Drug-induced liver injury due to antimicrobials, central nervous system agents, and nonsteroidal anti-inflammatory drugs. Semin Liver Dis. 2014; 34(2): 145-61. doi:10.1055/s-0034-1375956.
Al-Rejaie SS, Abuohashish HM, Al-Enazi MM, Al-Assaf AH, Parmar MY, Ahmed MM. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J Gastroenterol. 2013; 19(34): 5633-44. doi:10.3748/wjg.v19.i34.5633.
Collins HH, Cross AS, Dobek A, Opal SM, McClain JB, Sadoff JC. Oral ciprofloxacin and a infliximab to lipopolysaccharide protects leukopenic rats from lethal infection with Pseudomonas aeruginosa. J Infect Dis. 1989; 159(6): 1073-82. doi:10.1093/infdis/159.6.1073.
Witaicenis A, Luchini AC, Hiruma-Lima CA, Felisbino SL, Garrido-Mesa N, Utrilla P, et al. Suppression of TNBS-induced colitis in rats by 4-methylesculetin, a natural coumarin: comparison with prednisolone and sulphasalazine. Chem Biol Interact. 2012; 195(1): 76-85. doi:10.1016/j.cbi.2011.11.004.
Sandborn, W. J., & Hanauer, S. B. Infliximab in the treatment of Crohn’s disease: a user’s guide for clinicians. The American journal of gastroenterology. 2002; 97(12): 2962-72. doi.org/10.1111/j.1572-0241.2002.07093.x.
REITMAN S, FRANKEL S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957; 28(1): 56-63. doi:10.1093/ajcp/28.1.56.
Plummer D.T. An Introduction to Practical Biochemistry. 2nd Edition, London; McGraw-Hill: 1978: pp 144-145.
Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971; 31(1): 87-96. doi:10.1016/0009-8981(71)90365-2
Tietz, N.W. Clinical Guide to Laboratory Tests (ELISA). 3rd Edition, Philadelphia; W.B. Saunders, Co: 1995: pp 22-23.
Stocks J, Dormandy TL. The autoxidation of human red cell lipids induced by hydrogen peroxide. BJ Haem. 1971; 20(1): 95-111.
Bergmeyer HU, Editor. Methods of enzymatic analysis. Elsevier; 2012.
Schiff ER, Maddrey WC, Reddy KR, editors. Schiff’s Diseases of the Liver. John Wiley & Sons; 2017.
Kalas MA, Chavez L, Leon M, Taweesedt PT, Surani S. Abnormal liver enzymes: A review for clinicians. World J Hepatol. 2021; 13(11): 1688.
Vimalraj S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene. 2020; 754: 144855. doi:10.1016/j.gene.2020.144855.
Lowe D, Sanvictores T, Zubair M, John S. Alkaline phosphatase. StatPearls. 2023.
Losurdo, G., Brescia, I. V., Lillo, C., Mezzapesa, M., Barone, M., Principi, M., et al. Liver involvement in inflammatory bowel disease: What should the clinician know?. World J Hepatol. 2021; 13(11): 1534-51. doi:10.4254/wjh.v13.i11.1534.
Björnsson HK, Björnsson ES. Hepatotoxicity in inflammatory bowel disease: Immunomodulators, biologics, and beyond. Clin Liver Dis (Hoboken). 2024; 23(1): e0199. Published 2024 Jun 14. doi:10.1097/CLD.0000000000000199.
Owusu, G., Obiri, D. D., Ainooson, G. K., Osafo, N., Antwi, A. O., Duduyemi, B. M., et al. Acetic Acid-Induced Ulcerative Colitis in Sprague Dawley Rats Is Suppressed by Hydroethanolic Extract of Cordia vignei Leaves through Reduced Serum Levels of TNF-α and IL-6. Int J Chronic Dis. 2020; 2020: 8785497. Published 2020 Feb 6. doi:10. 1155/2020/8785497.
Abraham B, Quigley EMM. Ciprofloxacin and probiotics in inflammatory bowel disease: when to use them? [published correction appears in Frontline Gastroenterol. 2020;11(3): e1. doi: 10.1136/flgastro-2018-101057corr1]. Frontline Gastroenterol. 2020; 11(1): 62-9. doi:10.1136/flgastro-2018-101057.
Jeuring, S. F. G., Biemans, V. B. C., van den Heuvel, T. R. A., Zeegers, M. P., Hameeteman, W. H., Romberg-Camps, M. J. L., et al. Corticosteroid sparing in Inflammatory bowel disease is more often achieved in the immunomodulator and biological Era-results from the Dutch populationbased IBDSL cohort. Am J Gastroenterol. 2018; 113(3): 384-95. https://doi.org/10.1038/ajg.2017.482.
Sands BE, Hanauer SB, Colombel JF, Sandborn W, Schreiber S, Danese S, Klopocka M, Kulynych R, Kierkus J, Soltysiak A, Smolinski P. P492 Subcutaneous infliximab (CT-P13 SC) as maintenance therapy for ulcerative colitis: a phase 3, randomized, placebo-controlled study: results of the LIBERTY-UC study. J Crohn’s Colitis. 2023; 17(Supplement_1): i623-4.
Jarmakiewicz-Czaja S, Ferenc K, Filip R. Antioxidants as protection against reactive oxidative stress in inflammatory bowel disease. Metabolites. 2023; 13(4): 573.
Balestrieri P, Ribolsi M, Guarino MP, Emerenziani S, Altomare A, Cicala M. Nutritional aspects in inflammatory bowel diseases. Nutrients. 2020; 12(2): 372.
Vidal-Lletjós S, Beaumont M, Tomé D, Benamouzig R, Blachier F, Lan A. Dietary protein and amino acid supplementation in inflammatory bowel disease course: what impact on the colonic mucosa?. Nutrients. 2017; 9(3): 310.
Kalakonda A, Jenkins BA, John S. Physiology, Bilirubin. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
Gaspar R, Branco CC, Macedo G. Liver manifestations and complications in inflammatory bowel disease: A review. World J Hepatol. 2021; 13(12): 1956-67. doi:10.4254/wjh.v13.i12.1956.
Fu L, Qian Y, Shang Z, Sun X, Kong X, Gao Y. Ciprofloxacin enhancing drug-induced liver injury assessed for causality using Roussel Uclaf causality assessment method: Emerging role of gut microbiota dysbiosis. Front Med (Lausanne). 2022; 9: 972518. doi:10.3389/fmed.2022.972518.