Performance of line probe assay and phenotypic drug susceptibility testing in detecting drug-resistant tuberculosis
Main Article Content
Abstract
Background: Tuberculosis (TB) still threatens human beings when drug-resistant tuberculosis (DR-TB), such as rifampicin-resistant TB, isoniazid-resistant TB, multidrug-resistant TB (MDR-TB), pre-extensively drug-resistant TB, and extensively drug-resistant TB increases continuously. The drug susceptibility testing (DST) is important to detect DR-TB for TB treatment.
Objectives: The study aimed to assess first-line line probe assay (FL-LPA) performance of screening MDR-TB and detecting DR-TB on phenotypic drug susceptibility testing.
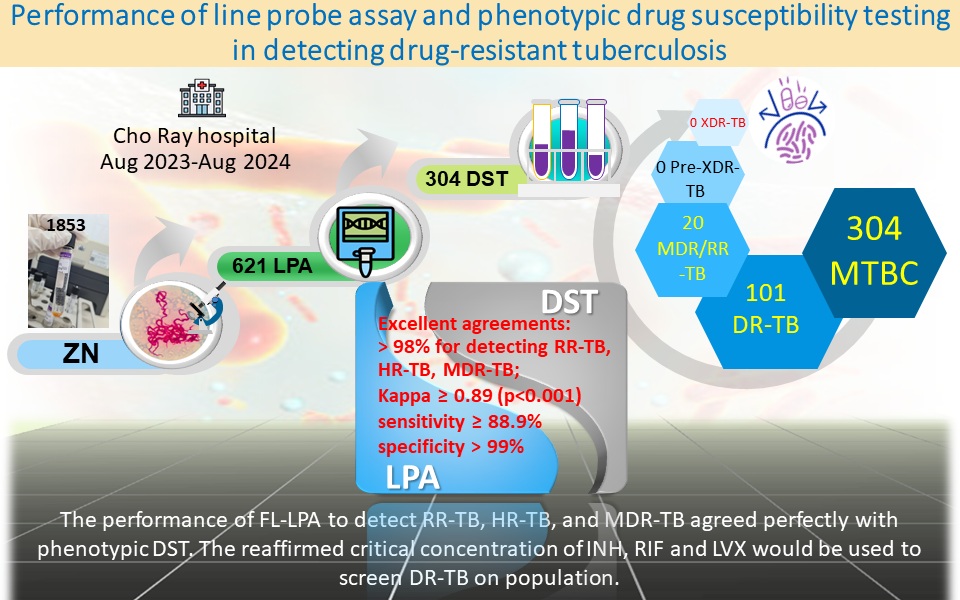
Materials and methods: A laboratory-based study was performed at Cho Ray Hospital from August 2023 to August 2024. The sputum samples of presumptive TB were inoculated in Mycobacterium growth indicator tube (MGIT). Positive inoculum was examined in acid-fast bacilli (AFB) by Ziehl-Neelsen microscope. Cord-forming AFB were yielded to FL-LPA to identify Mycobacterium tuberculosis complex (MTBC); detect rifampicin-resistant TB, isoniazid-resistant TB, and MDR-TB. The identified MTBC was subjected to FL phenotypic DST (performed by BACTEC MGIT 960) with SIRE kit, considering gold standard to assess FL-LPA performance. The detected multidrug and/or rifampicin-resistant TB (MDR/RR-TB) were subjected to the second- line MGIT DST including ethionamide, amikacin, levofloxacin, and linezolid to screen pre-extensively drug-resistant TB and extensively drug-resistant TB.
Results: Among 1853 samples inoculated, 621 positive MGIT tubes seen cord-forming AFB on Ziehl-Neelsen smear were performed to FL-LPA. Out of 621 LPA tests, 304 MTBC (61 isoniazid-resistant TB, 20 rifampicin-resistant TB, and 243 susceptible TB) were detected and compared to FL phenotypic DST. The excellent agreements between FL-LPA and FL phenotypic DST for detecting rifampicin-resistant TB, isoniazid- resistant TB, and MDR-TB were greater than 98%; kappa at 0.89 and above (p<0.001); with sensitivity values at 88.9% and above; specificity values at greater than 99%. For FL-MGIT DST, 101 (33.2%) were drug-resistant to at least one anti-TB agent, 81 (26.6%) to streptomycin, 60 (19.7%) to isoniazid, 20 (6.6%) to rifampicin. Among 20 MDR/RR-TB (2 rifampicin mono-resistant-TB and 18 MDR-TB) performed second line phenotypic DST, 25% resistance to ethionamide, and 100% susceptibility to amikacin, levofloxacin, and linezolid.
Conclusion:The performance of FL-LPA to detect rifampicin-resistant TB, isoniazid- resistant TB, and MDR-TB agreed perfectly with phenotypic DST. The reaffirmed critical concentration of isoniazid, rifampicin and levofloxacin would be used to screen DR-TB on population.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Stephenson J. WHO Report: Years of Progress in Global Tuberculosis Upset by COVID-19 Pandemic. JAMA Health Forum. 2022; 3(11): e224994. doi: 10.1001/jamahealthforum.2022.4994.
World Health Organization. WHO operational handbook on tuberculosis. Module 3: Diagnosis - Rapid diagnostics for tuberculosis detection, third edition. Geneva: World Health Organization. 2024. Licence: CC BY-NC-SA 3.0 IGO. Available from: https://iris.who.int/bitstream/handle/10665/ 376155/9789240089501-eng.pdf?sequence=1.
World Health Organization. Technical Report on critical concentrations for drug susceptibility testing of isoniazid and the rifamycins (rifampicin, rifabutin and rifapentine). Geneva: World Health Organization. 2021. Licence: CC BY-NC-SA 3.0 IGO. Available from: https://www.who.int/publications/i/ item/9789240017283.
World Health Organization. WHO consolidated guideline on tuberculosis. Module 3: Diagnosis - rapid diagnosis for tuberculosis detection, 2021 update. Geneva: World Health Organization. 2021. Licence: CC BY-NC-SA 3.0 IGO. Available from: https://www.who.int/publishcations/i/item/9889240029415.
Naidoo K, Perumal R, Ngema SL, Shunmugam L, Somboro AM. Rapid Diagnosis of Drug-Resistant Tuberculosis-Opportunities and Challenges. Pathogens. 2023; 13(1). doi: 10.3390/pathogens13010027.
Hain Lifescience. GenoType MTBDRplus VER 2 - Instruction for use [Internet]. 2015 [cited 2025 April 29]. Available from: https://www.hain-lifescience. de/include_datei/kundenmodule/packungsbeilage/ download.php?id=936.
Traore AN, Rikhotso MC, Mphaphuli MA, Patel SM, Mahamud HA, Kachienga LO, et al. Isoniazid and Rifampicin Resistance-Conferring Mutations in Mycobacterium tuberculosis Isolates from South Africa. Pathogens. 2023; 12(8). doi: 10.3390/pathogens 12081015.
World Health Organization. WHO operational handbook on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment, 2022 update. Geneva: World Health Organization. 2022. Available from: https://iris.who.int/bitstream/handle/ 10665/365333/9789240065116-eng.pdf?sequence=1.
Sinha P, Jacobson KR, Horsburgh CR, Jr., AcunaVillaorduna C. At Long Last: Short, All-Oral Regimens for Multidrug-Resistant Tuberculosis in the United States. Open Forum Infect Dis. 2023; 10(4): ofad177. doi: 10.1093/ofid/ofad177.
World Health Organization. Manual for selection of molecular WHO-recommended rapid diagnostic tests for detection of tuberculosis and drugresistant tuberculosis. Geneva: World Health Organization. 2022. Licence: CC BY-NC-SA 3.0 IGO. Available from: https://www.who.int/publications/i/ item/9789240042575.
Wrohan I, Nguyen TA, Nguyen VN, Nguyen BH, Hoang TTT, Nguyen PC, et al. Predictors of treatment outcomes among patients with multidrug-resistant tuberculosis in Vietnam: a retrospective cohort study. BMC Infect Dis. 2022; 22(1): 68. doi: 10.1186/ s12879-021-06992-x.
World Health Organization. WHO announces updated definitions of extensively drug-resistant tuberculosis. Geneva: World Health Organization. 2021 (cited 2025 Feb 13). Available from: https://www.who. int/news/item/27-01-2021-who-announcesupdated-definitions-of-extensively-drug-resistanttuberculosis.
World Health Organization. Laboratory Diagnosis of Tuberculosis by Sputum Microscopy- The Handbook. SA Pathology. 2013. Available from: https://www. stoptb.org/sites/default/files/imported/document/ TB_MICROSCOPY_HANDBOOK_FINAL.pdf.
Mannan A, Iram S, Ahmad A, Hussain S, Ahmad BM. Identification of TBc: Using MTP 64 protein and cord formation. J Pak Med Assoc. 2017; 67(10): 1600-3. Available from: https://www.academia.edu/ 92703961/Identification_of_TBc_Using_MTP_64_ protein_and_cord_formation.
Pinhata JMW, Felippe IM, Gallo JF, Chimara E, Ferrazoli L, de Oliveira RS. Growth characteristics of liquid cultures increase the reliability of presumptive identification of Mycobacterium tuberculosis complex. J Med Microbiol. 2018; 67(6): 828-33. doi: 10.1099/ jmm.0.000734.
World Health Organization. Technical manual for drug susceptibility testing of medicines used in the treatment of tuberculosis. Geneva: World Health Organization. 2018. Licence: CC BY-NC-SA 3.0 IGO. Available from: https://www.who.int/publications/i/ item/9789241514842.
Nguyen H, Nguyen H, Ha D, Huong D, Trung V, Ngoc K, et al. Rifampicin resistant Mycobacterium tuberculosis in Vietnam, 2020-2022. J Clin Tuberc Other Mycobact Dis. 2024; 35: 100431. doi: 10.1016/j.jctube.2024.100431.
European Centre for Disease Prevention and Control. Handbook on tuberculosis laboratory diagnostic methods in the European Union - updated 2022. Stockholm, STHML: European Centre for Disease Prevention and Control. 2023. doi: 10.2900/433652.
Albert H, Bwanga F, Mukkada S, Nyesiga B, Ademun JP, Lukyamuzi G, et al. Rapid screening of MDR-TB using molecular Line Probe Assay is feasible in Uganda. BMC Infect Dis. 2010; 10: 41. doi: 10.1186/14712334-10-41.
Pisal NS, Shah NC, Gandhi NN, Rao AS, Dedania MS, Pisal NS. A 1-year comparative evaluation of clinical performance of conventional direct composite restoration technique with a novel “custom shield” technique in class I compound lesions - A randomized clinical study. J Conserv Dent. 2022; 25(2): 135-9. doi: 10.4103/jcd.jcd_309_21.
Puyen ZM, Acosta J, Obregon G, Pacheco E, Ramirez H, Mendoza A, et al. Use and evaluation of a line probe assay in patients with tuberculosis in Peru: 2011-2013. Rev Panam Salud Publica [serial online]. 2016; 39(1): 19-25. Available from: https://www. ncbi.nlm.nih.gov/pubmed/27754534.
Meaza A, Kebede A, Yaregal Z, Dagne Z, Moga S, Yenew B, et al. Evaluation of genotype MTBDRplus VER 2.0 line probe assay for the detection of MDRTB in smear positive and negative sputum samples. BMC Infect Dis. 2017; 17(1): 280. doi: 10.1186/ s12879-017-2389-6. [23] Maningi NE, Malinga LA, Antiabong JF, Lekalakala RM, Mbelle NM. Comparison of line probe assay to BACTEC MGIT 960 system for susceptibility testing of first and second-line anti-tuberculosis drugs in a referral laboratory in South Africa. BMC Infect Dis. 2017; 17(1): 795. doi: 10.1186/s12879-017-2898-3.
Gunther G, Saathoff E, Rachow A, Ekandjo H, Diergaardt A, Marais N, et al. Clinical Evaluation of a Line-Probe Assay for Tuberculosis Detection and Drug-Resistance Prediction in Namibia. Microbiol Spectr. 2022; 10(3): e0025922. doi: 10.1128/ spectrum.00259-22.
Mahomed S, Mlisana K, Cele L, Naidoo K. Discordant line probe genotypic testing vs culture-based drug susceptibility phenotypic testing in TB endemic KwaZulu-Natal: Impact on bedside clinical decision making. J Clin Tuberc Other Mycobact Dis. 2020; 20: 100176. doi: 10.1016/j.jctube.2020.100176.
Hussain S, Sultan S, Riaz S, Hussain H, Javed H, Mazhar R. Synergy of Xpert (MTB/RIF) and Line probe assay for detection of rifampicin resistant strains of Mycobacterium tuberculosis. J Infect Dev Ctries. 2024; 18(8): 1241-8. doi: 10.3855/jidc.18945.
Yadav RN, Kumar Singh B, Sharma R, Chaubey J, Sinha S, Jorwal P. Comparative Performance of Line Probe Assay (Version 2) and Xpert MTB/RIF Assay for Early Diagnosis of Rifampicin-Resistant Pulmonary Tuberculosis. Tuberc Respir Dis (Seoul). 2021; 84(3): 237-44. doi: 10.4046/trd.2020.0171.
Raizada N, Sachdeva KS, Chauhan DS, Malhotra B, Reddy K, Dave PV, et al. A multi-site validation in India of the line probe assay for the rapid diagnosis of multi-drug resistant tuberculosis directly from sputum specimens. PLoS One. 2014; 9(2): e88626. doi: 10.1371/journal.pone.0088626.
Yigzaw WB, Torrelles JB, Wang SH, Tessema B. Magnitude of Phenotypic and MTBDRplus Line Probe Assay First-Line Anti-Tuberculosis Drug Resistance Among Tuberculosis Patients; Northwest Ethiopia. Infect Drug Resist. 2021; 14: 497-505. doi: 10.2147/ IDR.S292058.
Wondale B, Medhin G, Abebe G, Tolosa S, Mohammed T, Teklu T, et al. Phenotypic and genotypic drug sensitivity of Mycobacterium tuberculosis complex isolated from South Omo Zone, Southern Ethiopia. Infect Drug Resist. 2018; 11: 1581-9. doi: 10.2147/ IDR.S165088.
Madukaji L, Okohu I, Usman S, Oyedum U, Enagi A, Usman A, et al. Early detection of Pre-XDR TB with line probe assay in a high TB burden country. Afr Health Sci. 2021; 21(3): 968-74. doi: 10.4314/ahs. v21i3.2.
Huyen MN, Tiemersma EW, Lan NT, Cobelens FG, Dung NH, Sy DN, et al. Validation of the GenoType MTBDRplus assay for diagnosis of multidrug resistant tuberculosis in South Vietnam. BMC Infect Dis. 2010; 10: 149. doi: 10.1186/1471-2334-10-149.
Callum J, Nguyen PTB, Martinez E, Nguyen VT, Garden F, Nguyen NV, et al. Prevalence and genetic basis of first-line drug resistance of Mycobacterium tuberculosis in Ca Mau, Vietnam. ERJ Open Res. 2022; 8(4). doi: 10.1183/23120541.00122-2022.
Tutik K, Kristin PD, Yulia DS, Soedarsono. Discordance between Genexpert, line probe assay and drug susceptibility testing in assessing Drug-resistant tuberculosis. Indian J Forensic Medicine & Toxicology. 2021; 15(1): 1613-20. doi: https://doi.org/10.37506/ ijfmt.v15i1.13642.
World Health Organization Global Tuberculosis Programme. Rapid communication: key changes to the treatment of drug-resistant tuberculosis. Geneva, GE: World Health Organization. 2022 [cited 2025 Feb 13]. Available from: https://www.who.int/ publications/i/item/WHO-UCN-TB-2022-2.
Podlekareva DN, Folkvardsen DB, Skrahina A, Vassilenko A, Skrahin A, Hurevich H, et al. Tuberculosis Drug Susceptibility, Treatment, and Outcomes for Belarusian HIV-Positive Patients with Tuberculosis: Results from a National and International Laboratory. Tuberc Res Treat. 2021; 2021: 6646239. doi: 10.1155/2021/6646239.