In vitro anti–leukemia activity of Gynostemma pentaphyllum (Thunb.) on HL-60 leukemia cell line
Main Article Content
Abstract
Objective: This study aimed to investigate the in vitro anti-leukemia activities of the active extract derived from the leaves of Gynostemma pentaphyllum, focusing on its cytotoxic properties, cell cycle regulation, and impact on key protein markers involved in leukemia cell proliferation.
Materials and methods: G. pentaphyllum fractional extracts were prepared through sequential extraction using hexane, ethyl acetate, and ethanol solvents through maceration. The cytotoxicity of these extracts was determined against HL-60 leukemia cell line using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The most cytotoxic extract was further fractionated through column chromatography. The anti-leukemia activities of the active extract and its active fraction were evaluated through cell cycle analysis using flow cytometry. Western blotting was performed to assess the expression levels of key proliferation markers, Feline McDonough Sarcoma (FMS)-like tyrosine kinase 3 (FLT3) and Wilms’ tumor 1 (WT1) proteins.
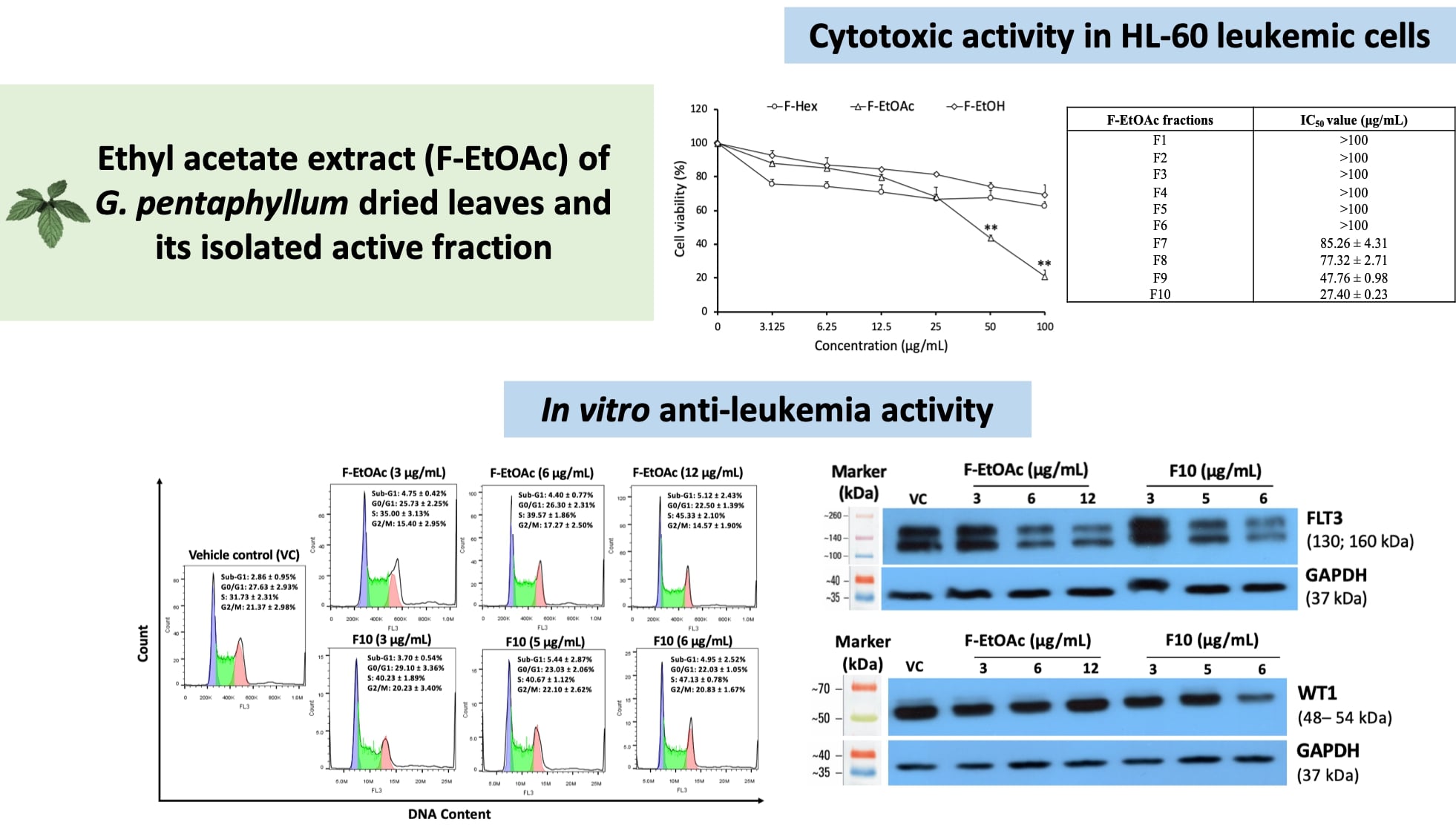
Results: The intermediate-polar ethyl acetate fractional extract (F-EtOAc) was found to be highly cytotoxic against HL-60 leukemia cells, with an IC50 value of 43.41±2.75 μg/mL. Among the isolated fractions of F-EtOAc, fraction F10 was identified as the most active. It was shown that F-EtOAc and F10 significantly induced S-phase cell cycle arrest and reduced the expression levels of FLT3 and WT1 proteins in HL-60 cells in a dose-dependent manner (p <0.001), suggesting that anti-proliferative molecular pathways contribute to their anti-leukemia potential.
Conclusion: This study’s findings indicate that the ethyl acetate extract of G. pentaphyllum contains bioactive compounds with anti-leukemia potential, supporting further investigation into its underlying molecular mechanisms.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia, Blood. 2016; 127: 2391-405. doi: 10.1182/blood-2016-03-643544.
Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019; 36: 70-87. doi: 10.1016/j.blre.2019.04.005.
Ryan MM. Acute Promyelocytic Leukemia: A Summary. J Adv Pract Oncol. 2018; 9(2): 178-87.
Grafone T, Palmisano M, Nicci C, Storti S. An overview on the role of FLT3-tyrosine kinase receptor in acute myeloid leukemia: biology and treatment. Oncol Rev. 2012; 6(1): e8. doi: 10.4081/oncol.2012.e8.
Hosen N, Shirakata T, Nishida S, Yanagihara M, Tsuboi A, Kawakami M, et al. The Wilms’ tumor gene WT1- GFP knock-in mouse reveals the dynamic regulation of WT1 expression in normal and leukemic hematopoiesis. Leukemia. 2007; 21: 1783-91. doi: 10.1038/sj.leu.2404752.
Burnett AK, Russell NH, Hills RK, Hunter AE, Kjeldsen L, Yin J, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol. 2013; 31(27): 3360-8. doi: 10.1200/JCO. 2012.47.4874.
Herranz-López M, Losada-Echeberría M, BarrajónCatalán E. The multitarget activity of natural extracts on cancer: Synergy and xenohormesis. Medicines. 2019; 6(1): 6. doi: 10.3390/medicines6010006.
Phumthum M, Balslev H, Barfod AS. Important Medicinal Plant Families in Thailand. Front Pharmacol. 2019; 10: 1125. doi: 10.3389/fphar.2019.01125.
Li Y, Lin W, Huang J, Xie Y, Ma W. Anti-cancer effects of Gynostemma pentaphyllum (Thunb.) Makino (Jiaogulan). Chinese Medicine. 2016; 11(43): 1-16. doi: 10.1186/s13020-016-0114-9.
Hsu HY, Yang JS, Lu KW, Yu CS, Chou ST. An experimental study on the antileukemia effects of gypenosides in vitro and in vivo. Integr Cancer Ther. 2011; 10(1): 101-12. doi: 10.1177/1534735410377198.
Ahmed A, Saleem MA, Saeed F, Afzaal M, Imran A, Nadeem M, et al. Gynostemma pentaphyllum an immortal herb with promising therapeutic potential: a comprehensive review on its phytochemistry and pharmacological perspective. Int J Food Prop. 2023; 26(1): 808-32. doi. org/10.1080/10942912.2023.218 5566.
Zhao Z, Guo Y, Wei H, Ran Q, Gu W. Predictions of the Potential Geographical Distribution and Quality of a Gynostemma pentaphyllum Base on the Fuzzy Matter Element Model in China. Sustainability. 2017; 9: 1114. doi.org/10.3390/su9071114.
Zhao X, Ge W, Miao Z. Integrative metabolomic and transcriptomic analyses reveals the accumulation patterns of key metabolites associated with flavonoids and terpenoids of Gynostemma pentaphyllum (Thunb.) Makino. Sci Rep. 2024; 14: 8644. doi.org/10.1038/ s41598-024-57716-5.
Lin JJ, Hsu HY, Yang JS, Lu KW, Wu RSC, Wu KC, et al. Molecular evidence of anti-leukemia activity of gypenosides on human myeloid leukemia HL-60 cells in vitro and in vivo using a HL-60 cells murine xenograft model. Phytomedicine. 2011; 18: 1075-85. doi: 10.1016/j.phymed.2011.03.009.
Panyajai P, Viriyaadhammaa N, Tima S, Chiampanichayakul S, Dejkriengkraikul P, Okonogi S, et al. Anticancer activity of Curcuma aeroginosa essential oil and its nano-formulations: cytotoxicity, apoptosis and cell migration effects. BMC Complement Med Ther. 2024; 24(1): 16. doi.org/10.1186/s12906-023- 04261-9.
Ellithey MS, Lall N, Hussein AA, Meyer D. Cytotoxic and HIV-1 enzyme inhibitory activities of Red Sea marine organisms. BMC Complement Altern Med. 2014; 14: 77. doi.org/10.1186/1472-6882-14-77.
Ahmad B, Khan S, Nabi G, Gamallat Y, Su P, Jamalat Y, Duan P, Yao L. Natural gypenosides: targeting cancer through different molecular pathways. Cancer Manag Res. 2019; 11: 2287-97. doi: 10.2147/CMAR. S185232.
Hoang TC, Nguyen MT, Nguyen TQ, Ho BTQ, Nguyen HT, Ngo TPD, Tran HNK, Bui TKL. In vitro anti-leukemia, antioxidant, and anti-inflammatory properties of Lantana camara. Braz J Biol. 2024; 84: e279899. doi: 10.1590/1519-6984.279899.
Mujtaba A, Masud T, Ahmad A, Naqvi SMS, Qazalbash MA, Levin RE. Effect of solvents on extraction yield, total flavonoid, total phenolic contents, DPPH scavenging activity and antibacterial potential of three apricot cultivars. Transylv Rev. 2016; 10: 1662-76.
Wang TX, Shi MM, Jiang JG. Bioassay-guided isolation and identification of anticancer and antioxidant compounds from Gynostemma pentaphyllum (Thunb.) Makino. RSC Adv. 2018;8(41):23181–23190. doi: 10.1039/ c8ra02803f.
Shi L, Cao JQ, Zhao H, Zhao YQ. A new triterpene saponin from Gynostemma pentaphyllum. Chin Herb Med. 2010; 2(4): 317-20. doi: 10.3969/j.issn.1674- 6384.2010.04.010.
Emmanuel O, Okeke SN, Dike RED, Bello AE, Ahuchaogu AA, Elekwachi C, Iwuchukwu BO. Role of plant-derived compounds in immune enhancement against uncontrollable cell proliferation. Brain Behavior and Immunity Integrative. 2024; 8: 100088. doi: 10. 1016/j.bbii.2024.100088.
Zhang X, Shi G, Wu X, Zhao Y. Gypensapogenin H from hydrolyzate of total Gynostemma pentaphyllum saponins induces apoptosis in human breast carcinoma cells. Nat Prod Res. 2020; 34(11): 1642-6. doi: 10.1080/14786419.2018.1525370.
Lu HF, Chen YS, Yang JS, Chen JC, Lu KW, Chiu TH, et al. Gypenosides induced G0/G1 arrest via inhibition of cyclin E and induction of apoptosis via activation of caspases-3 and -9 in human lung cancer A-549 cells. In Vivo. 2008; 22(2): 215-21.
Chen JC, Lu KW, Lee JH, Yeh CC, Chung JG. Gypenosides induced apoptosis in human colon cancer cells through the mitochondria-dependent pathways and activation of caspase-3. Anticancer Res. 2006; 26(6B): 4313-26.
Cheng TC, Lu JF, Wang JS, Lin LJ, Kuo HI, Chen BH. Antiproliferation effect and apoptosis mechanism of prostate cancer cell PC-3 by flavonoids and saponins prepared from Gynostemma pentaphyllum. J Agric Food Chem. 2011; 59(20): 11319-29. doi: 10.1021/ jf2018758.
Zhang XS, Zhao C, Tang WZ, Wu XJ, Zhao YQ. Gypensapogenin H, a novel dammarane-type triterpene induces cell cycle arrest and apoptosis on prostate cancer cells. Steroids. 2015; 104: 276-83. doi: 10.1016/ j.steroids.2015.10.014.
Strober W. Trypan Blue Exclusion Test of Cell Viability. Curr Protoc Immunol. 2015; 111: A3.B.1-A3.B.3. doi: 10.1002/0471142735.ima03bs111.