Hepatotoxic effects of sildenafil-containing “Tiger King” herbal supplement in a rat model: An in vitro and in vivo study
Main Article Content
Abstract
Background: The global market for erectile dysfunction (ED) treatments has seen a rise in herbal supplements marketed as natural alternatives to prescription medications. However, many of these products contain undeclared pharmaceutical ingredients, posing significant health risks.
Objective: This study aimed to investigate the chemical composition and potential hepatotoxic effects of “Tiger King,” a purported Chinese herbal supplement for sexual enhancement, using both in vitro and in vivo experiments.
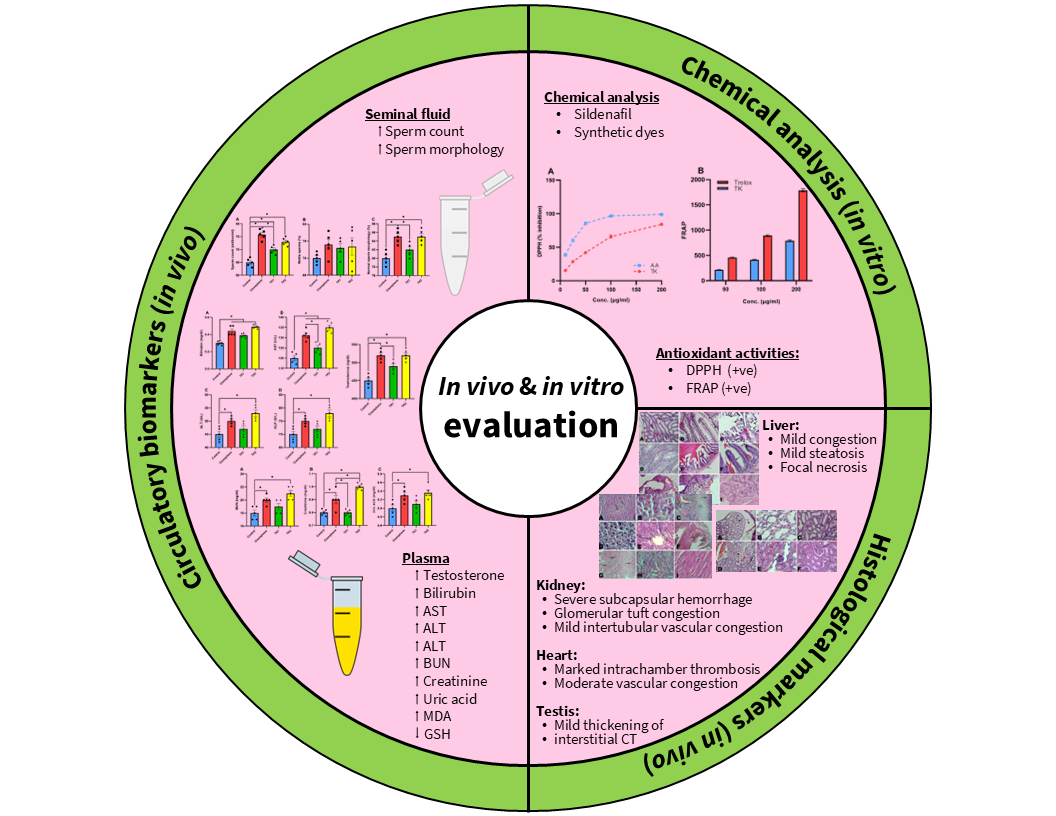
Materials and methods: Chemical analysis of “Tiger King” tablets was performed using thin-layer chromatography and colorimetric tests. Antioxidant activities were evaluated using DPPH and FRAP assays. In vivo studies were conducted using male Wistar rats (N=20) divided into four groups: control, clomiphene, low-dose “Tiger King” (5 mg/kg) or TK1, and high-dose “Tiger King” (10 mg/kg) or TK2. Treatments were administered orally for 30 days. Serum testosterone levels, sperm parameters, oxidative stress markers, liver and kidney function tests, and histopathological changes were assessed.
Results: Chemical analysis revealed the presence of sildenafil in “Tiger King” tablets, with no detectable amounts of the claimed herbal ingredients. In vivo studies showed significant increases in sperm count and testosterone levels in treated groups. However, oxidative stress markers (MDA, GSH) were significantly altered, and liver function tests (ALT, AST, ALP, bilirubin) were elevated in treatment groups with ALT increased by 17.8% (from 45.0±1.1 to 53.0±1.1 U/L), AST by 12.5% (from 120.0±1.1 to 135.0±1.1 U/L) in the high-dose TK2 group. Histopathological examination revealed mild to moderate changes in liver, kidney, and reproductive organs of treated animals, including hepatic steatosis, renal glomerular congestion, and glandular atrophy in reproductive tissues.
Conclusion: This study provides evidence that “Tiger King” contains undeclared sildenafil and lacks the advertised herbal components. Its use is associated with improved reproductive parameters but also with significant biochemical and histopathological changes which draw attention to potential health risks of adulterated herbal supplements.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Petre GC, Francini-Pesenti F, Vitagliano A, Grande G, Ferlin A, Garolla A. Dietary supplements for erectile dysfunction: Analysis of marketed products, systematic review, meta-analysis and rational use. Nutrients. 2023; 15(17): 3677. doi: 10.3390/ nu15173677
Nissan R, Poperno A, Y. Stein G, et al. A case of hepatotoxicity induced by adulterated “Tiger King”, a Chinese herbal medicine containing sildenafil. Curr Drug Saf. 2016; 11(2): 184-8. doi: 10.2174/157 4886311207040257
FDA. Public Notification: Tiger King contains hidden drug ingredient.; 2014. https://www.fda.gov/drugs/ medication-health-fraud/public-notification-tigerking-contains-hidden-drug-ingredient
Australian Government Department of Health. Tiger King Tablets.; 2015. http://www.tga.gov.au/alert/tiger-king-tablets
Reeuwijk NM, Venhuis BJ, de Kaste D, Hoogenboom LAP, Rietjens IMCM, Martena MJ. Sildenafil and analogous phosphodiesterase type 5 (PDE-5) inhibitors in herbal food supplements sampled on the Dutch market. Food Addit Contam Part A. 2013; 30(12): 2027-34. doi: 10.1080/19440049.2013.848294
Low MY, Zeng Y, Li L, et al. Safety and quality assessment of 175 illegal sexual enhancement products seized in red-light districts in Singapore. Drug Saf. 2009; 32(12): 1141-6. doi: 10.2165/11316690- 000000000-00000
Venhuis BJ, Zwaagstra ME, Keizers PHJ, de Kaste D. Dose-to-dose variations with single packages of counterfeit medicines and adulterated dietary supplements as a potential source of false negatives and inaccurate health risk assessments. J Pharm Biomed Anal. 2014; 89: 158-65. doi: 10.1016/j.jpba.2013.10.038
Qahtan Mohammed B, Ali Hussaini H, Adnan abdulhameed W. The effect of aspirin and sildenafil on endometrial thickness, oocyte characteristic, embryo quality and pregnancy test in iraqi infertile women undergoing intracytoplasmic sperm injection. IraQi J Embryos Infertil Res. 2022; 12(2): 40-61. doi: 10.28969/IJEIR.v12.i2.r4.22
State of Israel Ministry of Health. Counterfeit Medicines.; 2023. https://www.health.gov.il/English/ Topics/PharmAndCosmetics/pharm_crime/Pages/ default.aspx
Wolfhagen FHJ, Vermeulen HG, de Man RA, Lesterhuis W. Initially obscure hepatotoxicity attributed to sildenafil. Eur J Gastroenterol Hepatol. 2008; 20(7): 710-2. doi: 10.1097/MEG.0b013e3282f2bbb5
Daghfous R, El Aidli S, Zaiem A, Loueslati MH, Belkahia C. Sildenafil-associated hepatotoxicity. Am J Gastroenterol. 2005; 100(8): 1895-6. doi: 10.1111/ j.1572-0241.2005.41983_6.x
Enomoto M, Sakaguchi H, Ominami M, et al. Sildenafilinduced severe cholestatic hepatotoxicity. Am J Gastroenterol. 2009; 104(1): 254-5. doi: 10.1038/ajg. 2008.18
Graziano S, Montana A, Zaami S, et al. Sildenafilassociated hepatoxicity: a review of the literature. Eur Rev Med Pharmacol Sci. 2017; 21(Suppl1): 17-22. http://www.ncbi.nlm.nih.gov/pubmed/28379598
Al-Maliki RS. COVID-19 vaccination doesn’t influence sperm motility, concentration, and morphology Rehab. J Assoc Med Sci. 2025; 58(1): 185-91. doi: 10. 12982/JAMS.2025.02
Patel DN, Li L, Kee CL, Ge X, Low MY, Koh HL. Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: Analytical techniques and challenges. J Pharm Biomed Anal. 2014; 87: 176-90. doi: 10.1016/j.jpba.2013.04.037
Venhuis BJ, de Kaste D. Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: A history, analytical aspects and health risks. J Pharm Biomed Anal. 2012; 69: 196-208. doi: 10.1016/j.jpba.2012.02.014
Abourashed E, Abdel-Kader M, Habib AA. HPTLC determination of sildenafil in pharmaceutical products and aphrodisiac herbal preparations. J Planar Chromatogr – Mod TLC. 2005; 18(105): 372-6. doi: 10.1556/JPC.18.2005.5.7
Wagner H, Bladt S. Plant Drug Analysis: A Thin Layer Chromatography Atlas. 2nd Ed. Springer-Verlag Berlin Heidelberg; 1996.
V. Le A, E. Parks S, H. Nguyen M, D. Roach P. Improving the vanillin-sulphuric acid method for quantifying total saponins. Technologies. 2018; 6(3): 84. doi: 10.3390/technologies6030084
Sherma J, Fried B, eds. Handbook of Thin-Layer Chromatography. 3rd Ed. Marcel Dekker; 1991.
Rebane R, Leito I, Yurchenko S, Herodes K. A review of analytical techniques for determination of Sudan I–IV dyes in food matrixes. J Chromatogr A. 2010; 1217(17): 2747-57. doi: 10.1016/j.chroma.2010.02.038
Savaliya AA, Shah RP, Prasad B, Singh S. Screening of Indian aphrodisiac ayurvedic/herbal healthcare products for adulteration with sildenafil, tadalafil and/or vardenafil using LC/PDA and extracted ion LC–MS/TOF. J Pharm Biomed Anal. 2010; 52(3): 406- 9. doi: 10.1016/j.jpba.2009.05.021
Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci Technol. 1995; 28(1) :25-30. doi: 10.1016/S0023-6438(95)80008-5
Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996; 239(1): 70-6. doi: 10.1006/abio.1996.0292
Janjic MM, Stojkov NJ, Bjelic MM, Mihajlovic AI, Andric SA, Kostic TS. Transient Rise of Serum Testosterone Level After Single Sildenafil Treatment of Adult Male Rats. J Sex Med. 2012; 9(10): 2534-43. doi: 10.1111/j.1743-6109.2012.02674.x
Saraiva KLA, Silva AKSE, Wanderley MI, De Araújo AA, De Souza JRB, Peixoto CA. Chronic treatment with sildenafil stimulates Leydig cell and testosterone secretion. Int J Exp Pathol. 2009; 90(4): 454-62. doi: 10.1111/j.1365-2613.2009.00660.x
Spitzer M, Bhasin S, Travison TG, Davda MN, Stroh H, Basaria S. Sildenafil increases serum testosterone levels by a direct action on the testes. Andrology. 2013; 1(6): 913-8. doi: 10.1111/j.2047-2927.2013.00131.x
Andric SA, Janjic MM, Stojkov NJ, Kostic TS. Sildenafil treatment in vivo stimulates Leydig cell steroidogenesis via the cAMP/cGMP signaling pathway. Am J Physiol Metab. 2010; 299(4): E544-50. doi: 10.1152/ajpendo.00337.2010