In vitro activity of ethanolic extract of propolis (EEP) against Candida albicans pathogenicity mechanisms
Main Article Content
Abstract
Background: Candidiasis is an opportunistic fungal infection mainly caused by an overgrowth of Candida albicans. Several virulence factors, including the ability to change its morphology from yeast to hyphae and the secretion of hydrolytic enzymes, contribute to and promote the pathogenesis of the disease.
Objective: We aimed to investigate the efficacy of ethanolic extract of propolis (EEP) on growth and some major virulence factors of C. albicans that involved pathogenesis development, such as adhesion, hyphal germination, invasion, and virulence enzyme activities.
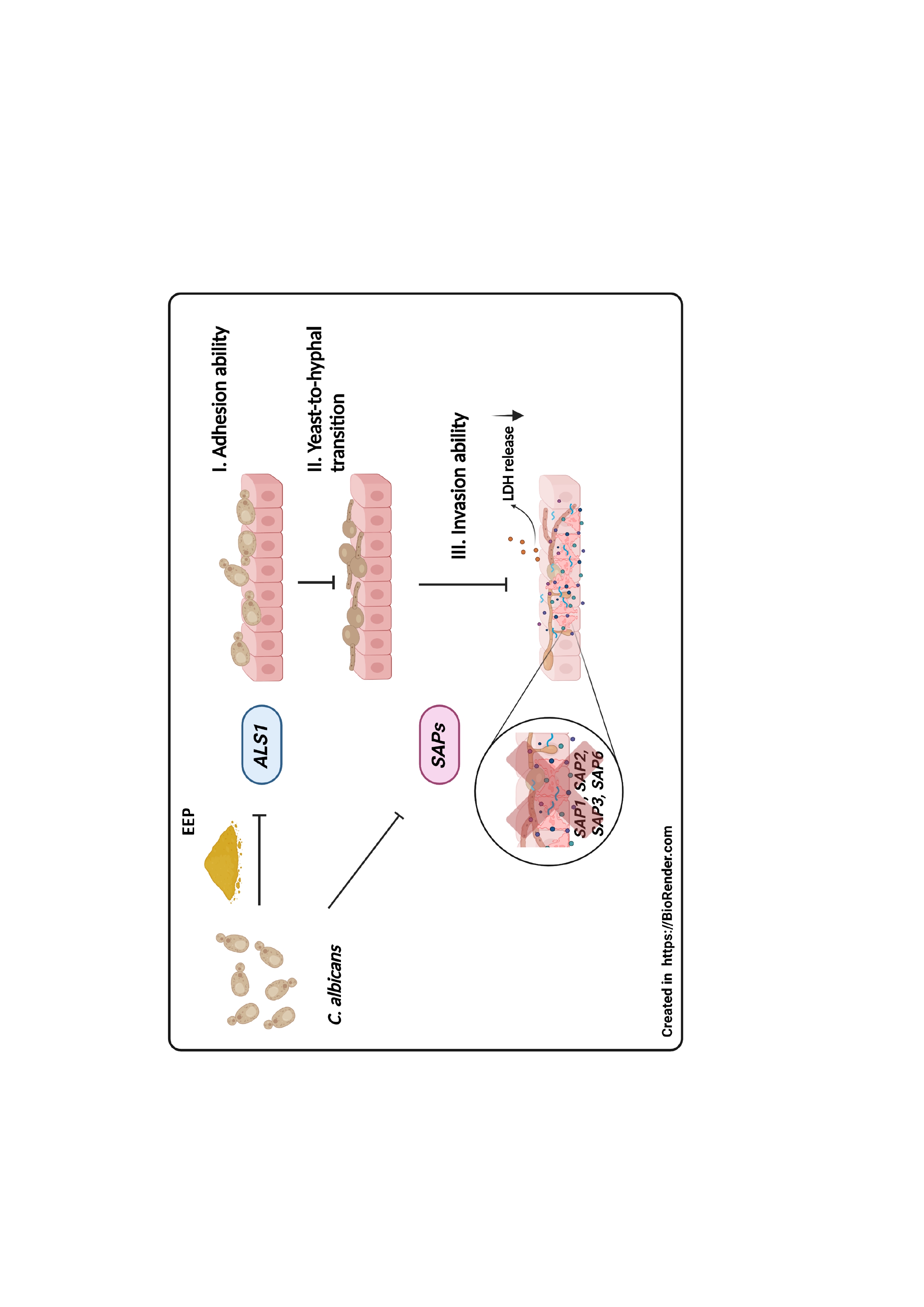
Materials and methods: C. albicans DMST 21424 was treated with various concentrations of EEP, and the growth of yeast cells was determined by colonyforming unit assay. An investigation of morpho-transformation ability was carried out using a hyphal germination assay and a hyphal length measurement. The adhesion ability of EEP-treated yeast cells was determined on both abiotic and biotic materials using acrylic discs and HeLa cell surfaces, respectively. The infected cells were treated with EEP to study invasion ability, and the cell damage was indicated by lactate dehydrogenase (LDH) activity assay. The effect of EEP on virulence enzyme activity was evaluated on sheep blood agar for hemolysin, egg yolk agar for phospholipase, and bovine serum albumin (BSA) agar for proteinase. Then, the mRNA expression levels of virulence enzyme-related genes such as ALS1, SAP1, SAP2, SAP3, and SAP6 were assessed by quantitative reverse transcriptase polymerase chain reaction (RT-PCR).
Results: The growth of EEP-treated yeasts was suppressed in a dose-dependent manner. The yeast-to-hyphae transition property was significantly reduced in EEP-treated yeast. EEP treatment also significantly decreased adhesion, invasion, and proteinase activity. However, there was no difference in hemolysin and phospholipase activities between EEP-treated yeast and the control. Moreover, EEP also remarkably down-regulated agglutinin-like-sequence 1 (ALS1) and secreted aspartyl proteinase (SAPs) genes.
Conclusion: The findings revealed that EEP exhibited potent anti-C. albicans virulence factors associated with pathogenesis. Therefore, this study suggested that propolis might be an effective complementary medicine alternative for candidiasis treatment.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Macias-Paz IU, Perez-Hernandez S, Tavera-Tapia A, Luna-Arias JP, Guerra-Cardenas JE, Reyna-Beltran E. Candida albicans the main opportunistic pathogenic fungus in humans. Rev Argent Microbiol. 2023; 55(2): 189-98. doi: 10.1016/j.ram.2022.08.003
Talapko J, Juzbasic M, Matijevic T, Pustijanac E, Bekic S, Kotris I, Skrlec I, et al. Candida albicans-The virulence factors and clinical manifestations of infection. J Fungi (Basel). 2021; 7(2): 79. doi: 10.3390/ jof7020079
da Silva Dantas A, Lee KK, Raziunaite I, Schaefer K, Wagener J, Yadav B, et al. Cell biology of Candida albicans-host interactions. Curr Opin Microbiol. 2016; 34: 111-8. doi: 10.1016/j.mib.2016.08.006
Weissman Z, Kornitzer D. A family of Candida cell surface haem-binding proteins involved in haemin and haemoglobin-iron utilization. Mol Microbiol. 2004; 53(4): 1209-20. doi: 10.1111/j.1365-2958.2004. 04199.x
Wiederhold NP. Pharmacodynamics, mechanisms of action and resistance, and spectrum of activity of new antifungal agents. J Fungi (Basel). 2022; 8(8): 875. doi: 10.3390/jof8080857
Queiroga MC, Laranjo M, Andrade N, Marques M, Costa AR, Antunes CM. Antimicrobial, antibiofilm and toxicological assessment of propolis. Antibiotics (Basel). 2023; 12(2): 347. doi: 10.3390/antibiotics 12020347
Sangboonruang S, Semakul N, Sookkree S, Kantapan J, Ngo-Giang-Huong N, Khamduang W, et al. Activity of propolis nanoparticles against HSV-2: Promising approach to inhibiting infection and replication. Molecules. 2022; 27(8): 2560. doi: 10.3390/molecules 27082560
Kietrungruang K, Sookkree S, Sangboonruang S, Semakul N, Poomanee W, Kitidee K, et al. Ethanolic extract propolis-loaded niosomes diminish phospholipase B1, biofilm formation, and intracellular replication of Cryptococcus neoformans in macrophages. Molecules. 2023; 28(17): 6224. doi: 10.3390/molecules 28176224
Iadnut A, Mamoon K, Thammasit P, Pawichai S, Tima S, Preechasuth K, et al. In vitro antifungal and antivirulence activities of biologically synthesized ethanolic extract of propolis-loaded PLGA nanoparticles against Candida albicans. Evid Based Complement Alternat Med. 2019; 2019: 3715481. doi: 10.1155/ 2019/3715481
Wanasaengsakul S, Khongkhawithun P, Tienthong T. In vitro efficacy of polident in reducing candida biofilm on surface of acrylic resin. J Dent Assoc Thai. 2008; 58(3): 178-88.
Borg-von Zepelin M, Wagner T. Fluorescence assay for the detection of adherent Candida yeasts to target cells in microtest plates. Mycoses. 1995; 38(9-10): 339-47. doi: 10.1111/j.1439-0507.1995. tb00062.x
Alves CT, Wei XQ, Silva S, Azeredo J, Henriques M, Williams DW. Candida albicans promotes invasion and colonisation of Candida glabrata in a reconstituted human vaginal epithelium. J Infect. 2014; 69(4): 396- 407. doi: 10.1016/j.jinf.2014.06.002
Tsang CSP, Chu FCS, Leung WK, Jin LJ, Samaranayake LP, Siu SC. Phospholipase, proteinase and haemolytic activities of Candida albicans isolated from oral cavities of patients with type 2 diabetes mellitus. J Med Microbiol. 2007; 56(Pt 10): 1393-8. doi: 10.1099/jmm.0.47303-0
Al-Abeid HM, Abu-Elteen KH, Elkarmi AZ, Hamad MA. Isolation and characterization of Candida spp. in Jordanian cancer patients: prevalence, pathogenic determinants, and antifungal sensitivity. Jpn J Infect Dis. 2004; 57(6): 279-84.
Li Y, Ma Y, Zhang L, Guo F, Ren L, Yang R, et al. In vivo inhibitory effect on the biofilm formation of Candida albicans by liverwort derived riccardin D. PLoS One. 2012; 7(4): e35543.
Schaller M, Korting HC, Schafer W, Bastert J, Chen W, Hube B. Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol Microbiol. 1999; 34(1): 169-80. doi: 10.1046/j.1365-2958.1999.01590.x
Sforcin JM. Biological Properties and Therapeutic Applications of Propolis. Phytother Res. 2016; 30(6): 894-905. doi: 10.1002/ptr.5605
Zabaiou N, Fouache A, Trousson A, Baron S, Zellagui A, Lahouel M, et al. Biological properties of propolis extracts: Something new from an ancient product. Chem Phys Lipids. 2017; 207(Pt B): 214-22. doi: 10. 1016/j.chemphyslip.2017.04.005
Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011; 133(2): 253-60. doi: 10.1016/j.jep.2010. 10.032
Khacha-ananda S, Saenphet K, Saenphet S, Tragoolpua K, ChantawannakulS P, Tragoolpua Y. Evaluation of the stability of propolis granule and toxicity study in wistar rats. Chiang Mai J Science. 2018; 45(1): 162-76.
Fikri AM, Sulaeman A, Marliyati SA, Fahrudin M, Handharyani E. Effect of propolis on maternal toxicity pharmaceutical sciences asia. 2021; 48(3): 224-30. doi: 10.29090/psa.2021.03.20.056
Silva JC, Rodrigues S, Feas X, Estevinho LM. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem Toxicol. 2012; 50(5): 1790-5. doi: 10.1016/j.fct.2012.02.097
Kiran Kumar Reddy G, Hari Kumar P, Padmavathi AR, Kutala VK, Sandur SK, Nancharaiah YV. Antifungal and antibiofilm action of triphenylphosphoniumconjugated curcumin on Candida albicans: Efficacy and activity mechanisms. Int Biodeterioration & Biodegradation. 2024; 189: 105751. doi: 10.1016/j. ibiod.2024.105751
Anjum SI, Ullah A, Khan KA, Attaullah M, Khan H, Ali H, et al. Composition and functional properties of propolis (bee glue): A review. Saudi J Biol Sci. 2019; 26(7): 1695-703. doi: 10.1016/j.sjbs.2018.08.013
de Castro PA, Bom VL, Brown NA, de Almeida RS, Ramalho LN, Savoldi M, et al. Identification of the cell targets important for propolis-induced cell death in Candida albicans. Fungal Genet Biol. 2013; 60: 74-86. doi: 10.1016/j.fgb.2013.07.001
Li ZJ, Liu M, Dawuti G, Dou Q, Ma Y, Liu HG, et al. Antifungal activity of gallic acid in vitro and in vivo. Phytother Res. 2017; 31(7): 1039-45. doi: 10.1002/ ptr.5823
Kim JH, Haff RP, Faria NC, Martins Mde L, Chan KL, Campbell BC. Targeting the mitochondrial respiratory chain of Cryptococcus through antifungal chemosensitization: A model for control of non-fermentative pathogens. Molecules. 2013; 18(8): 8873-94. doi: 10.3390/molecules18088873
Pereira Rangel L, Fritzen M, Yunes RA, Leal PC, Creczynski-Pasa TB, Ferreira-Pereira A. Inhibitory effects of gallic acid ester derivatives on Saccharomyces cerevisiae multidrug resistance protein Pdr5p. FEMS Yeast Res. 2010; 10(3): 244-51. doi: 10.1111/j.1567- 1364.2010.00603.x
Willaert RG. Adhesins of yeasts: Protein structure and interactions. J Fungi (Basel). 2018; 4(4): 119. doi: 10.3390/jof4040119
Gomaa OM, Gaweesh AS. Variation in adhesion and germ tube formation of oral Candida using Egyptian propolis. Can J Microbiol. 2013; 59(3): 197-203. doi: 10.1139/cjm-2012-0374
Martin H, Kavanagh K, Velasco-Torrijos T. Targeting adhesion in fungal pathogen Candida albicans. Future Med Chem. 2021; 13(3): 313-34. doi: 10.4155/ fmc-2020-0052
Fu Y, Ibrahim AS, Sheppard DC, Chen YC, French SW, Cutler JE, et al. Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol Microbiol. 2002; 44(1): 61-72. doi: 10.1046/j.1365-2958.2002.02873.x
Feldman M, Tanabe S, Howell A, Grenier D. Cranberry proanthocyanidins inhibit the adherence properties of Candida albicans and cytokine secretion by oral epithelial cells. BMC Complement Altern Med. 2012; 12: 6. doi: 10.1186/1472-6882-12-6
Ishida K, de Mello JC, Cortez DA, Filho BP, UedaNakamura T, Nakamura CV. Influence of tannins from Stryphnodendron adstringens on growth and virulence factors of Candida albicans. J Antimicrob Chemother. 2006; 58(5): 942-9. doi: 10.1093/jac/ dkl377
Silva-Carvalho R, Baltazar F, Almeida-Aguiar C. Propolis: A complex natural product with a plethora of biological activities that can be explored for drug development. Evid Based Complement Alternat Med. 2015; 2015: 206439. doi: 10.1155/2015/206439
Chen H, Zhou X, Ren B, Cheng L. The regulation of hyphae growth in Candida albicans. Virulence. 2020; 11(1): 337-48. doi: 10.1080/21505594.2020.1748930
Corrêa JL, Veiga FF, Jarros IC, Costa MI, Castilho PF, de Oliveira KMP, et al. Propolis extract has bioactivity on the wall and cell membrane of Candida albicans. J Ethnopharmacology. 2020; 256: 112791. doi: 10.10 16/j.jep.2020.112791
Schroter C, Hipler UC, Wilmer A, Kunkel W, Wollina U. Generation of reactive oxygen species by Candida albicans in relation to morphogenesis. Arch Dermatol Res. 2000; 292(5): 260-4. doi: 10.1007/s0040300 50484
Theberge S, Semlali A, Alamri A, Leung KP, Rouabhia M. C. albicans growth, transition, biofilm formation, and gene expression modulation by antimicrobial decapeptide KSL-W. BMC Microbiol. 2013; 13: 246. doi: 10.1186/1471-2180-13-246
Meenambiga SS, Venkataraghavan R, Biswal RA. In silico analysis of plant phytochemicals against secreted aspartic proteinase enzyme of Candida albicans. J Appl Pharm Sci. 2018; 8(11): 140-50. doi: 10.7324/ JAPS.2018.81120
Gupta P, Gupta S, Sharma M, Kumar N, Pruthi V, Poluri KM. Effectiveness of phytoactive molecules on transcriptional expression, biofilm matrix, and cell wall components of Candida glabrata and its clinical isolates. ACS Omega. 2018; 3(9): 12201-14. doi: 10.1021/ acsomega.8b01856
Tobaldini-Valerio FK, Bonfim-Mendonca PS, Rosseto HC, Bruschi ML, Henriques M, Negri M, et al. Propolis: A potential natural product to fight Candida species infections. Future Microbiol. 2016; 11: 1035-46. doi: 10.2217/fmb-2015-0016
Rhimi W, Aneke CI, Annoscia G, Otranto D, Boekhout T, Cafarchia C. Effect of chlorogenic and gallic acids combined with azoles on antifungal susceptibility and virulence of multidrug-resistant Candida spp. and Malassezia furfur isolates. Med Mycol. 2020; 58(8): 1091-101. doi: 10.1093/mmy/myaa010