Effect of pre-exposure to low-dose radiation followed by H2 O2 treatment on leukemic cells proliferation
Main Article Content
Abstract
Background: Leukemia is a blood cancer illness that causes morbidity and mortality around the world. A new approach to treatment is challenging.
Objective: The objective was to investigate the effects of pre-exposure to low-dose radiation (LDR) followed by H2 O2 treatment on cell proliferation in leukemic cells.
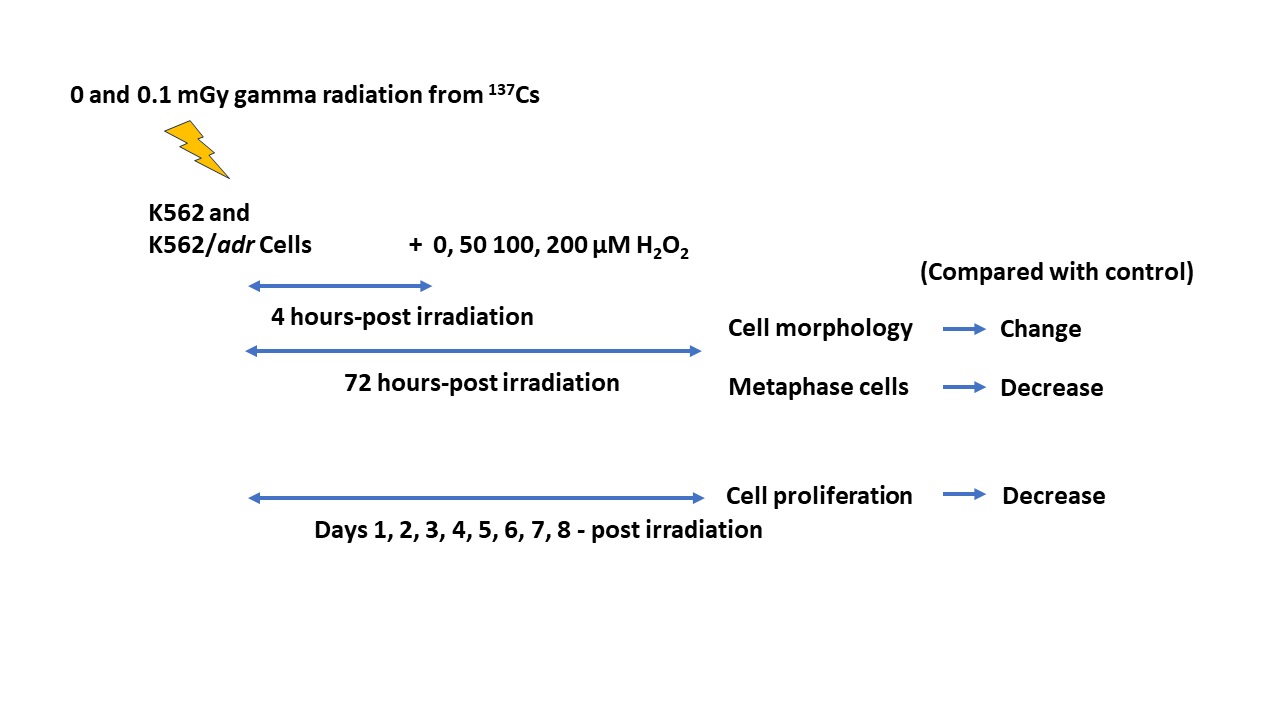
Materials and methods: The human leukemic doxorubicin-sensitive K562 and doxorubicin-resistant K562/adr cells were exposed to 0.1 mGy of gamma radiation from radioactive 137Cs at a dose rate of 0.001 Gy/min followed by treatments with various concentration of H2 O2 at 4 hrs-post irradiation. The cell morphology and metaphase cells in treated groups were compared with a control group at 72 hrs post-irradiation. The cell proliferation was determined at days 1-8 post irradiation.
Results: The results showed that the number of cells in treated groups with 50, 100, and 200 µM of H2 O2 was less than the control group (0 µM H2 O2 ). The pattern of the cells rapidly increased at 24-48 hrs and then decreased until the 5th and 6th days. This was found in all treated groups, except those treated with 200 µM of H2 O2 alone and with pre-exposure to LDR followed by H2 O2 groups. The microscopic images showed rough cells, shrinking cells, irregular-shaped cells, and swelling cells in H2 O2 alone and pre-exposure to LDR followed by H2 O2 groups. The metaphase cells were significantly decreased in H2 O2 alone groups in a concentration-dependent manner. In addition, the metaphase cells were also considerably reduced in pre-exposure to LDR, followed by H2 O2 groups when compared with control and LDR alone groups.
Conclusion: This data provides a basis for additional studies to help clarify the potential use and benefits of pre-exposure to LDR followed by H2 O2 treatment in cancer cells.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Verrax J, Taper H, Buc Calderon P. Targeting cancer cells by an oxidant-based therapy. Curr Mol Pharmacol. 2008; 1(1): 80-92. doi 10.2174/1874467210801010080.
Renschler MF. The emerging role of reactive oxygen species in cancer therapy. Eur J Cancer. 2004; 40(13): 1934-40. doi 10.1016/j.ejca.2004.02.031
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007; 39(1): 44-84. doi 10.1016/j. biocel.2006.07.001
Djavaheri-Mergny M, Wietzerbin J, Besançon F. 2-Methoxyestradiol induces apoptosis in Ewing sarcoma cells through mitochondrial hydrogen peroxide production. Oncogene. 2003; 22(17): 2558-67. doi 10.1038/sj.onc.1206356
Evens AM, Lecane P, Magda D, Prachand S, Singhal S, Nelson J, et al. Motexafin gadolinium generates reactive oxygen species and induces apoptosis in sensitive and highly resistant multiple myeloma cells. Blood. 2005; 105(3): 1265-73. doi 10.1182/blood2004-03-0964
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6): 394-424. doi 10.3322/caac.21492
Michieli M, Damiani D, Michelutti A, Candoni A, Masolini P, Scaggiante B, et al. Restoring uptake and retention of daunorubicin and idarubicin in P170- related multidrug resistance cells by low concentration D-verapamil, cyclosporin-A and SDZ PSC 833. Haematologica. 1994; 79(6): 500-7.
Xia Q, Wang ZY, Li HQ, Diao YT, Li XL, Cui J, et al. Reversion of p-glycoprotein-mediated multidrug resistance in human leukemic cell line by diallyl trisulfide. Evid Based Complement Alternat Med. 2012; 2012: 719805. doi doi 10.1155/2012/719805
Luckey TD. Physiological benefits from low levels of ionizing radiation. Health Phys. 1982; 43(6): 771-89. doi10.1097/00004032-198212000-00001
Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005; 78(925): 3-7. doi 10.1259/bjr/63353075
Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984; 223(4636): 594-7. doi 10.1126/science.6695170
Chen Z, Sakai K. Enhancement of radiation-induced apoptosis by preirradiation with low-dose X-rays in human leukemia MOLT-4 cells. J Radiat Res. 2004; 45(2): 239-43. doi 10.1269/jrr.45.239
Jiang H, Xu Y, Li W, Ma K, Cai L, Wang G. Low-dose radiation does not induce proliferation in tumor cells in vitro and in vivo. Radiat Res. 2008; 170(4): 477-87. doi 10.1667/rr1132.1
Li SJ, Liang XY, Li HJ, Yang GZ, Li W, Li Z, et al. Lowdose irradiation inhibits proliferation of the p53null type human prostate cancer cells through the ATM/ p21 pathway. Int J Mol Med. 2018; 41(1): 548-54. doi 10.3892/ijmm.2017.3237
Aye KT, Wattanapongpitak S, Supawat B, Kothan S, Udomtanakunchai C, Tima S, et al. Effect of prelow-dose irradiation on anticancer activities of gallic acid in leukemic K562 and K562/Dox cells: cell viability and cellular energetic state studies. Med Oncol. 2022; 39(12): 229. doi 10.1007/s12032-022- 01835-4
Aye KT, Wattanapongpitak S, Supawat B, Kothan S, Udomtanakunchai C, Tima S, et al. Gallic acid enhances pirarubicininduced anticancer in living K562 and K562/Dox leukemia cancer cells through cellular energetic state impairment and Pglycoprotein inhibition. Oncol Rep. 2021; 46(4): 227. doi 10.3892/ or.2021. 8178
Supawat B, Homnuan P, Kanthawong N, Semrasa N, Tima S, Kothan S, et al. Different responses of normal cells (red blood cells) and cancer cells (K562 and K562/Dox cells) to low-dose (137)Cs gamma-rays. Mol Clin Oncol. 2021; 14(4): 74. doi 10.3892/mco.2021.2236
Vilema-Enríquez G, Arroyo A, Grijalva M, AmadorZafra RI, Camacho J. Molecular and Cellular Effects of Hydrogen Peroxide on Human Lung Cancer Cells: Potential Therapeutic Implications. Oxid Med Cell Longev. 2016; 2016: 1908164. doi 10.1155/2016/ 1908164
Chen JP, Xu DG, Yu XY, Zhao FM, Xu DQ, Zhang X, et al. Discrepancy between the effects of morronside on apoptosis in human embryonic lung fibroblast cells and lung cancer A549 cells. Oncol Lett. 2014; 7(4): 927-32. doi 10.3892/ol.2014.1850
Su H, Liu DD, Zhao M, Hu WL, Xue SS, Cao Q, et al. Dual-Enzyme Characteristics of PolyvinylpyrrolidoneCapped Iridium Nanoparticles and Their Cellular Protective Effect against H2O2-Induced Oxidative Damage. ACS Appl Mater Interfaces. 2015; 7(15): 8233-42. doi 10.1021/acsami.5b01271
Chirino YI, Sánchez-Pérez Y, Osornio-Vargas AR, MoralesBárcenas R, Gutiérrez-Ruíz MC, Segura-García Y, et al. PM(10) impairs the antioxidant defense system and exacerbates oxidative stress driven cell death. Toxicol Lett. 2010; 193(3): 209-16. doi 10.1016/j.toxlet. 2010.01.009
Chiou SY, Lee YS, Jeng MJ, Tsao PC, Soong WJ. Moderate hypothermia attenuates oxidative stress injuries in alveolar epithelial A549 cells. Exp Lung Res. 2013; 39(6): 217-28. doi 10.3109/01902148.2013.792881
Oraki Kohshour M, Najafi L, Heidari M, Ghaffari Sharaf M. Antiproliferative effect of H2O2 against human acute myelogenous leukemia KG1 cell line. J Acupunct Meridian Stud. 2013; 6(3): 134-41. doi 10.1016/j.jams.2012.08.004
Datta K, Babbar P, Srivastava T, Sinha S, Chattopadhyay P. p53 dependent apoptosis in glioma cell lines in response to hydrogen peroxide induced oxidative stress. Int J Biochem Cell Biol. 2002; 34(2): 148-57. doi 10.1016/s1357-2725(01)00106-6
Barbouti A, Doulias PT, Nousis L, Tenopoulou M, Galaris D. DNA damage and apoptosis in hydrogen peroxide-exposed Jurkat cells: bolus addition versus continuous generation of H(2)O(2). Free Radic Biol Med. 2002;33(5):691-702. doi 10.1016/s0891-5849 (02)00967-x
Dumont A, Hehner SP, Hofmann TG, Ueffing M, Dröge W, Schmitz ML. Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-kappaB. Oncogene. 1999; 18(3): 747-57. doi 10.1038/sj.onc.1202325
Rithidech KN, Tungjai M, Whorton EB. Protective effect of apigenin on radiation-induced chromosomal damage in human lymphocytes. Mutat Res. 2005; 585(1-2): 96-104. doi 10.1016/j.mrgentox.2005.04.003
Tungjai M, Tubthaing N, Kothan S. Lysosomes of Cancerous and Normal cells in Response to Lowenergy/low-dose Medical Diagnostic X-rays. Bangladesh Journal of Medical Science. 2019;18(4):830-4. doi 10.3329/bjms.v18i4.42915