Fibroblast growth factors in female reproductive disorders: A systematic review
Main Article Content
Abstract
Background: Fibroblast Growth Factors (FGFs) are a family of signaling proteins that play key roles in various biological processes, including cell proliferation, differentiation, and tissue repair. In recent years, there has been growing interest in the role of FGFs in reproductive biology, particularly in the context of female fertility and assisted reproductive technologies (ART).
Objective: This systematic review aimed to evaluate the role of Fibroblast Growth Factors (FGFs) in female reproductive disorders with a focus on repeated implantation failure (RIF), diminished ovarian reserve (DOR), polycystic ovary syndrome (PCOS), and premature ovarian insufficiency (POI).
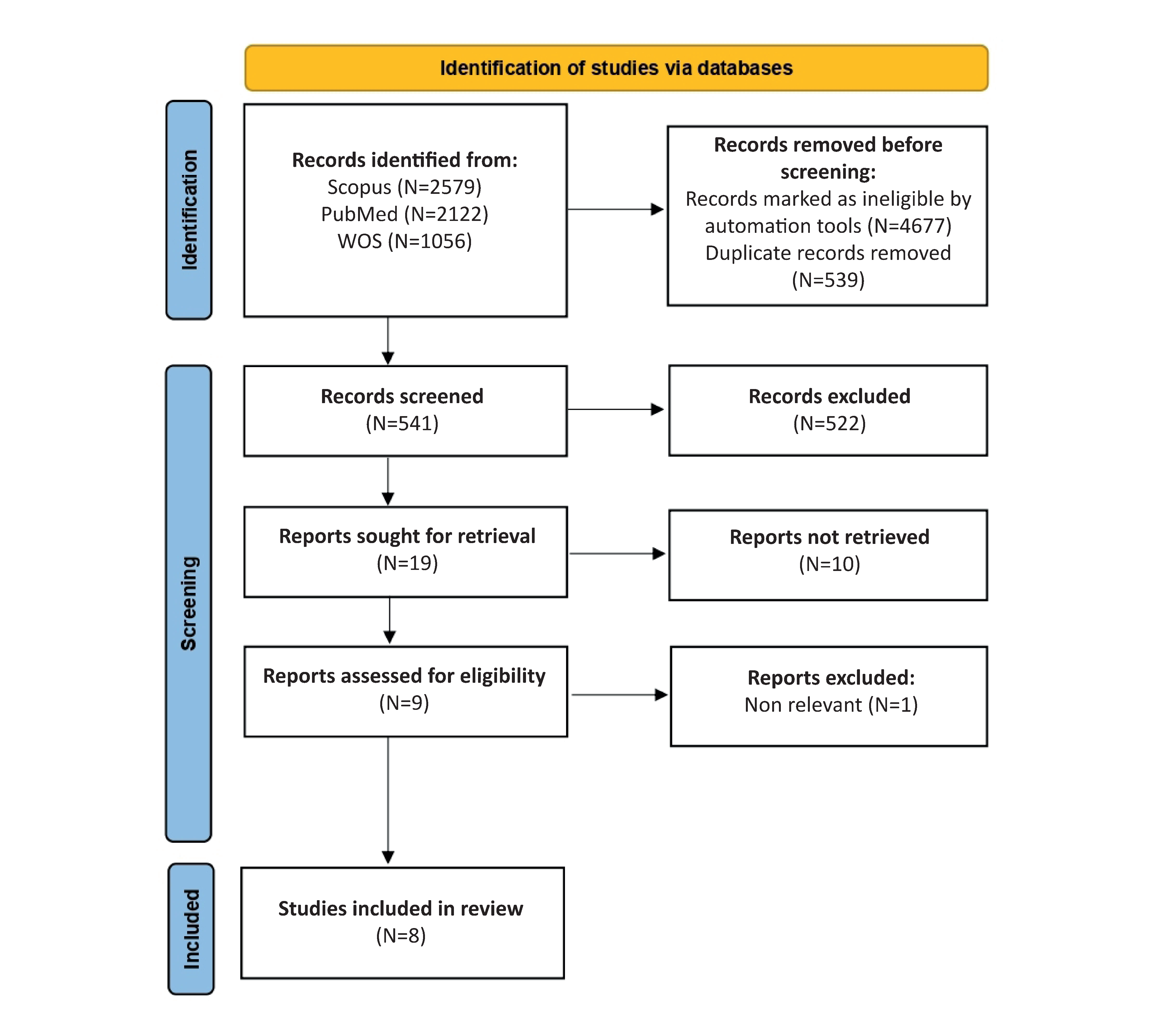
Materials and methods: A comprehensive literature search was conducted across PubMed, Web of Science, and Scopus databases for studies published between January 2014 and December 2024, yielding 8 eligible studies.
Results: The review found: reduced FGF-2 levels in PCOS patients’ serum and follicular fluid, contrasting with earlier research; lower serum FGF-1 levels and specific genetic variants in RIF patients; elevated FGF-13 levels associated with increased androgen levels and ovarian volume in PCOS; altered FGF-5 expression in DOR cases; and unexpectedly elevated FGF-2 levels in biochemical POI.
Conclusion: FGF isoforms may be a contributor to the pathophysiology of female reproductive disorders. However, these findings while important, the review identified important limitations including small sample sizes and the tendency to focus on individual FGF isoforms. For future studies, there’s a need for larger and more comprehensive studies examining the entire FGF family and their connections in reproductive dysfunction.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Farooq M, Khan AW, Kim MS, Choi S. The role of fibroblast growth factor (FGF) signaling in tissue repair and regeneration. Cells. 2021; 10(11): 3242. doi: 10.3390/cells10113242
Phan P, Saikia BB, Sonnaila S, et al. The saga of endocrine FGFs. Cells. 2021; 10(9): 2418. doi: 10.3390/cells10092418
Ardizzone A, Scuderi SA, Giuffrida D, et al. Role of fibroblast growth factors receptors (FGFRs) in brain tumors, focus on astrocytoma and glioblastoma. Cancers (Basel). 2020; 12(12): 3825. doi: 10.3390/cancers12123825
Vernon RK, Spicer LJ. Effects of basic fibroblast growth factor and heparin on follicle-stimulating hormone-induced steroidogenesis by bovine granulosa cells1. J Anim Sci. 1994; 72(10): 2696-702. doi: 10.2527/1994.72102696x
Abhari S, Lu J, Hipp HS, et al. A case-control study of follicular fluid cytokine profiles in women with diminished ovarian reserve. Reprod Sci. 2022; 29(9): 2515-24. doi: 10.1007/s43032-021-00757-2
Liu Y, Li S, Tao T, et al. Intrafollicular fibroblast growth factor 13 in polycystic ovary syndrome: relationship with androgen levels and oocyte developmental competence. J Ovarian Res. 2018; 11(1): 87. doi: 10.1186/s13048-018-0455-3
Owen BM, Bookout AL, Ding X,et al. FGF21 contributes to neuroendocrine control of female reproduction. Nat Med. 2013; 19(9): 1153-6. doi: 10.1038/nm.3250
Aghajanpour S, Mehraein F, Amjadi F, et al. Endometrial scratching in unexplained repeated implantation failure causes two competing forces, angiogenesis and anti-angiogenesis: An RCT study. Int J Reprod Biomed. 2024; 22(4): 253-68. doi: 10.18502/ijrm.v22i4. 16387
Kharamani A, Mashayekhi F, Salehi Z. Association of Fibroblast Growth Factor-1 Promoter Polymorphism and its Serum Concentrations with Repeated Implantation Failure after In vitro Fertilisation: A Cross-sectional Study. J Hum Reprod Sci. 2024; 17(2): 121-7. doi: 10.4103/jhrs.jhrs_68_24
Günther V, Otte S v., Freytag D, Maass N, Alkatout I. Recurrent implantation failure – an overview of
current research. Gynecol Endocrinol. 2021; 37(7): 584-90. doi: 10.1080/09513590.2021.1878136
McCallie BR, Haywood M, Denomme MM, et al. Forecasting early onset diminished ovarian reserve for young reproductive age women. J Assist Reprod Genet. 2021; 38(7): 1853-60. doi: 10.1007/s10815- 021-02155-8
Liu P, Zhang X, Hu J, et al. Dysregulated cytokine profile associated with biochemical premature ovarian insufficiency. Am J Reprod Immunol. 2020; 84(4). doi: 10.1111/aji.13292
Chon SJ, Umair Z, Yoon MS. Premature ovarian insufficiency: Past, present, and uture. Front Cell Dev Biol. 2021; 9. doi:10.3389/fcell.2021.672890
Touraine P, Chabbert-Buffet N, Plu-Bureau G, Duranteau L, Sinclair AH, Tucker EJ. Premature ovarian insufficiency. Nat Rev Dis Prim. 2024; 10(1):
doi: 10.1038/s41572-024-00547-5
Patil K, Hinduja I, Mukherjee S. Alteration in angiogenic potential of granulosa-lutein cells and follicular fluid contributes to luteal defects in polycystic ovary syndrome. Hum Reprod. 2021; 36(4): 1052-64. doi: 10.1093/humrep/deaa351
Dileep A, Samy MAF, Hussain N, Zain Alabdind S. Effect of weight loss on symptoms of polycystic ovarian syndrome among women of reproductive age. Dubai Med J. 2021; 4(2): 127-32. doi: 10.1159/000514025
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. Published online March 29, 2021: n71. doi: 10.1136/bmj.n71
Yland J, Carvalho LFP, Beste M, et al. Endometrioma, the follicular fluid inflammatory network and its association with oocyte and embryo characteristics. Reprod Biomed Online. 2020; 40(3): 399-408. doi: 10.1016/j.rbmo.2019.12.005
Vithoulkas A, Levanduski M, Goudas VT, Illmensee K. Co-culture of human embryos with autologous cumulus cell clusters and its beneficial impact of secreted growth factors on preimplantation development as compared to standard embryo culture in assisted reproductive technologies (ART). Middle East Fertil Soc J. 2017; 22(4): 317-22. doi: 10.1016/j.mefs.2017.05.009
LaVallee TM, Prudovsky IA, McMahon GA, Hu X, Maciag T. Activation of the MAP Kinase Pathway by FGF-1 Correlates with Cell Proliferation Induction While Activation of the Src Pathway Correlates with Migration. J Cell Biol. 1998; 141(7): 1647-58. doi: 10.1083/jcb.141.7.1647
Maffucci T, Raimondi C, Abu-Hayyeh S, et al. A Phosphoinositide 3-Kinase/Phospholipase Cgamma1 Pathway Regulates Fibroblast Growth FactorInduced Capillary Tube Formation. Hotchin NA, ed. PLoS One. 2009; 4(12): e8285. doi: 10.1371/journal. pone.0008285
Sahni M, Ambrosetti DC, Mansukhani A, Gertner R, Levy D, Basilico C. FGF signaling inhibits chondrocyte proliferation and regulates bone development through the STAT-1 pathway. Genes Dev. 1999; 13(11): 1361-6. doi: 10.1101/gad.13.11.1361
Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. WIREs Dev Biol. 2015; 4(3): 215-66. doi: 10.1002/wdev.176
Shimada M, Yamashita Y. The Key Signaling Cascades in Granulosa Cells During Follicular Development and Ovulation Process. J Mamm Ova Res. 2011; 28(1): 25-31. doi: 10.1274/jmor.28.25
Sobinoff AP, Sutherland JM, Mclaughlin EA. Intracellular signalling during female gametogenesis. MHR Basic Sci Reprod Med. 2013; 19(5): 265-78. doi: 10.1093/molehr/gas065
Li T, Mo H, Chen W, et al. Role of the PI3K-Akt Signaling Pathway in the Pathogenesis of Polycystic Ovary Syndrome. Reprod Sci. 2017; 24(5): 646-55. doi: 10.1177/1933719116667606
Artini PG, Monti M, Matteucci C, Valentino V, Cristello F, Genazzani AR. Vascular endothelial
growth factor and basic fibroblast growth factor in polycystic ovary syndrome during controlled ovarian hyperstimulation. Gynecol Endocrinol. 2006; 22(8): 465-70. doi: 10.1080/09513590600906607
He T, Liu Y, Zhao S, Liu H, Wang Z, Shi Y. Comprehensive assessment the expression of core elements related to IGFIR/PI3K pathway in granulosa cells of women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2019; 233: 134-40. doi: 10.1016/j.ejogrb. 2018.12.010
Hammadeh ME, Fischer-Hammadeh C, Hoffmeister H, et al. Fibroblast growth factor (FGF), intracellular adhesion molecule (sICAM-1) level in serum and follicular fluid of infertile women with polycystic ovarian syndrome, endometriosis and tubal damage, and their effect on ICSI outcome. Am J Reprod Immunol. Published online 2003; 50(2): 124-30. doi: 10.1034/j.1600-0897.2003.00056.x