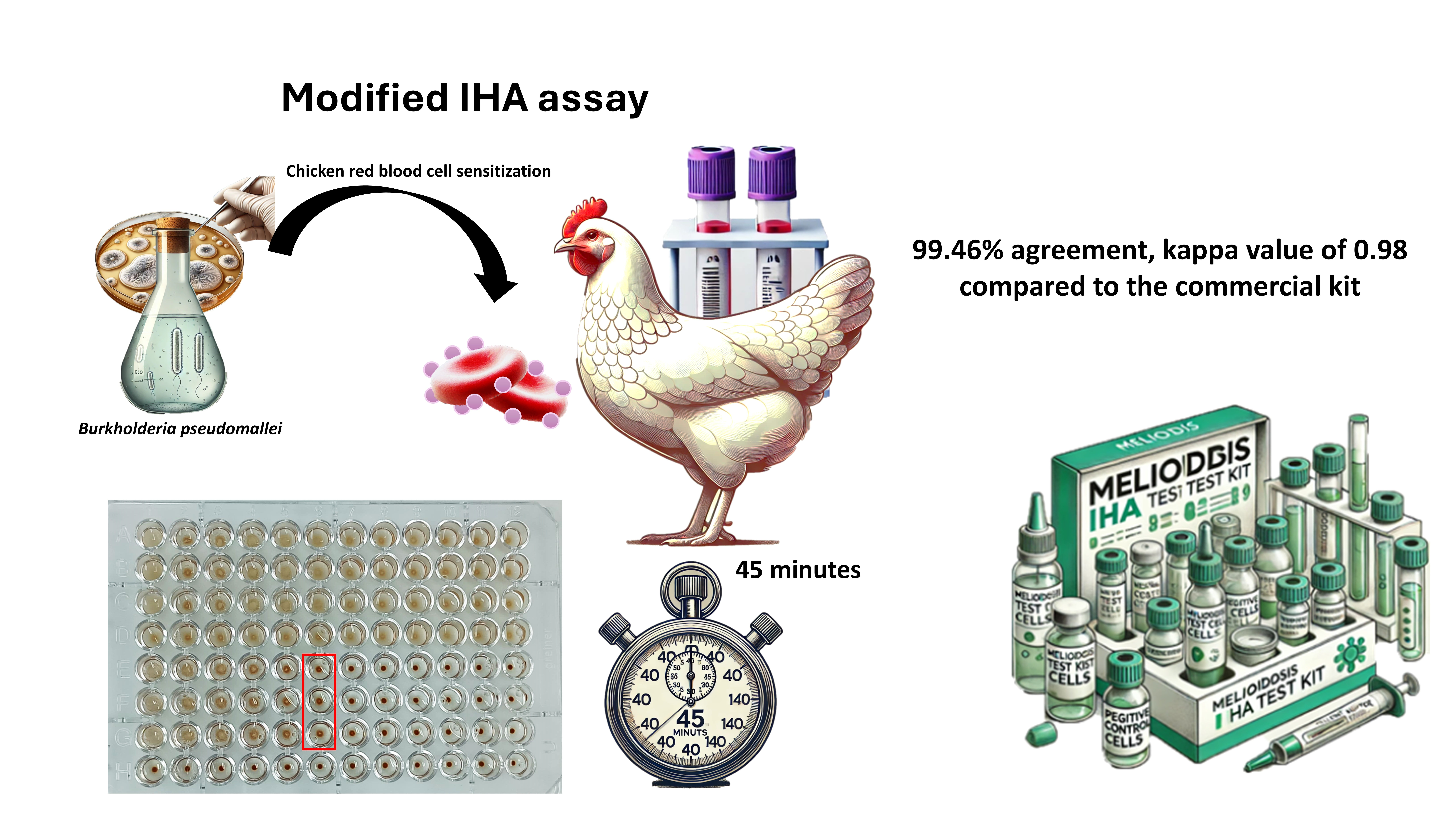
Evaluation of the modified-indirect hemagglutinin assay using chicken red blood cells as a routine melioidosis detection in Maharaj Nakorn Chiang Mai Hospital
Main Article Content
Abstract
Background: Melioidosis is a life-threatening illness caused by Burkholderia pseudomallei, which is endemic throughout Thailand. The indirect hemagglutination assay (IHA), a serological test, is widely used to diagnose melioidosis. However, the conventional IHA available in Thailand still requires more than 4 hours for measurement time, limiting its efficiency in clinical settings. This study addresses the need for a quicker and more efficient diagnostic method for melioidosis.
Objective: The study aimed to evaluate the agreement between a modified indirect hemagglutination (modified IHA) assay and the commercially available IHA kit for routine melioidosis diagnosis at Maharaj Nakorn Chiang Mai Hospital. Chicken red blood cells were used instead of sheep red blood cells to reduce diagnostic time and costs while maintaining high accuracy and reliability.
Materials and methods: A total of 368 serum samples were tested using the modified IHA assay, which utilized chicken red blood cells instead of sheep red blood cells used in the commercial IHA kit. The results were compared with those of the commercial IHA kit for melioidosis detection.
Results: The modified IHA assay showed 99.46% agreement, with an excellent kappa value of 0.98, compared to the commercial kit. In the validation step, the modified IHA assay correctly identified 100% (54 out of 54) of positive samples and 100% (54 out of 54) of negative samples. However, 0.63% (2 out of 320) false positives were observed in the diagnostic samples with the modified IHA assay.
Conclusion: The modified IHA assay may serve as a valuable alternative for the routine diagnosis of melioidosis because it is less time-consuming and more cost-effective than the commercial IHA kit. However, further studies with more clinical samples are warranted to confirm its utility.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Gassiep I, Armstrong M, Norton R. Human Melioidosis. Clin Microbiol Rev. 2020; 33(2). doi: 10.1128/CMR. 00006-19.
Hinjoy S, Hantrakun V, Kongyu S, Kaewrakmuk J, Wangrangsimakul T, Jitsuronk S, et al. Melioidosis in Thailand: Present and Future. Trop Med Infect Dis. 2018; 3(2): 38. doi: 10.3390/tropicalmed3020038.
Hantrakun V, Kongyu S, Klaytong P, Rongsumlee S, Day NPJ, Peacock SJ, et al. Clinical Epidemiology of 7126 Melioidosis Patients in Thailand and the Implications for a National Notifiable Diseases Surveillance System. Open Forum Infect Dis. 2019; 6(12): ofz498. doi: 10.1093/ofid/ofz498.
Hemarajata P, Baghdadi JD, Hoffman R, Humphries RM. Burkholderia pseudomallei: Challenges for the Clinical Microbiology Laboratory. J Clin Microbiol. 2016; 54(12): 2866-73. doi: 10.1128/JCM.01636-16.
Chaiwarith; R, Patiwetwitoon; P, Supparatpinyo; K, Sirisanthana T. Melioidosis at Maharaj Nakorn Chiang Mai Hospital, Thailand. J Infect Dis Antimicrob Agents. 2005; 22(2): 45-51.
Currie BJ, Fisher DA, Howard DM, Burrow JN, Selvanayagam S, Snelling PL, et al. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop. 2000; 74(2-3): 121-7. doi: 10.1016/s0001-706x(99)00060-1.
Ngauy V, Lemeshev Y, Sadkowski L, Crawford G. Cutaneous melioidosis in a man who was taken as a prisoner of war by the Japanese during World War II. J Clin Microbiol. 2005; 43(2): 970-2. doi: 10.1128/ JCM.43.2.970-972.2005.
Sheridan EA, Ramsay AR, Short JM, Stepniewska K, Wuthiekanun V, Simpson AJ. Evaluation of the Wayson stain for the rapid diagnosis of melioidosis. J Clin Microbiol. 2007; 45(5): 1669-70. doi: 10.1128/ JCM.00396-07.
Hoffmaster AR, AuCoin D, Baccam P, Baggett HC, Baird R, Bhengsri S, et al. Melioidosis diagnostic workshop, 2013. Emerg Infect Dis. 2015; 21(2): e141045. doi: 10.3201/eid2102.141045.
Cheng AC, Peacock SJ, Limmathurotsakul D, Wongsuvan G, Chierakul W, Amornchai P, et al. Prospective evaluation of a rapid immunochromogenic cassette test for the diagnosis of melioidosis in northeast Thailand. Trans R Soc Trop Med Hyg. 2006; 100(1): 64-7. doi: 10.1016/j.trstmh. 2005.04.019.
Hierholzer JC, Suggs MT. Standardized viral hemagglutination and hemagglutination-inhibition tests. I. Standardization of erythrocyte suspensions. Appl Microbiol. 1969; 18(5): 816-23. doi: 10.1128/ am.18.5.816-823.1969.
Pawar SD, Parkhi SS, Koratkar SS, Mishra AC. Receptor specificity and erythrocyte binding preferences of avian influenza viruses isolated from India. Virol J. 2012; 9: 251. doi: 10.1186/1743-422X-9-251.
Kaufmann L, Syedbasha M, Vogt D, Hollenstein Y, Hartmann J, Linnik JE, et al. An Optimized Hemagglutination Inhibition (HI) Assay to Quantify Influenza-specific Antibody Titers. J Vis Exp. 2017; 130: 55833. doi: 10.3791/55833.
Trombetta CM, Ulivieri C, Cox RJ, Remarque EJ, Centi C, Perini D, et al. Impact of erythrocyte species on assays for influenza serology. J Prev Med Hyg. 2018; 59(1): E1-E7. doi:10.15167/2421-4248/jpmh 2018.59.1.870.
Teparrukkul P. Indirect Hemagglutination Test for the Diagnosis of Meliodosis in Ubon Rachathani. J Infect Dis Antimicrob Agents. 1997; 14(1): 17-9.
Gassiep I, Bauer MJ, Harris PNA, Chatfield MD, Norton R. Laboratory Safety: Handling Burkholderia pseudomallei Isolates without a Biosafety Cabinet. J Clin Microbiol. 2021; 59(7): e0042421. doi: 10.1128/ JCM.00424-21.
Rodnan GP, Ebaugh FG, Jr., Fox MR. The life span of the red blood cell and the red blood cell volume in the chicken, pigeon and duck as estimated by the use of Na2 Cr51O4 , with observations on red cell turnover rate in the mammal, bird and reptile. Blood. 1957; 12(4): 355-66. doi:10.1111/j.1753-4887.1947. tb04174.x
Tucker EM. Red cell life span in young and adult sheep. Research in Veterinary Science. 1963; 4(1): 11-23. doi: 10.1016/S0034-5288(18)34879-3