Development and implementation of the external quality assessment program for erythrocyte sedimentation rate in hematology laboratories in Thailand
Main Article Content
Abstract
Background: The erythrocyte sedimentation rate (ESR) remains the most widely used screening test for monitoring the course of infections, inflammatory diseases, and some types of cancer. Proficiency Testing (PT) programs are unavailable in Thailand for ESR.
Objective: This study aimed to develop and initiate the PT program to improve testing quality through evaluation and analysis of the laboratory test results for ESR.
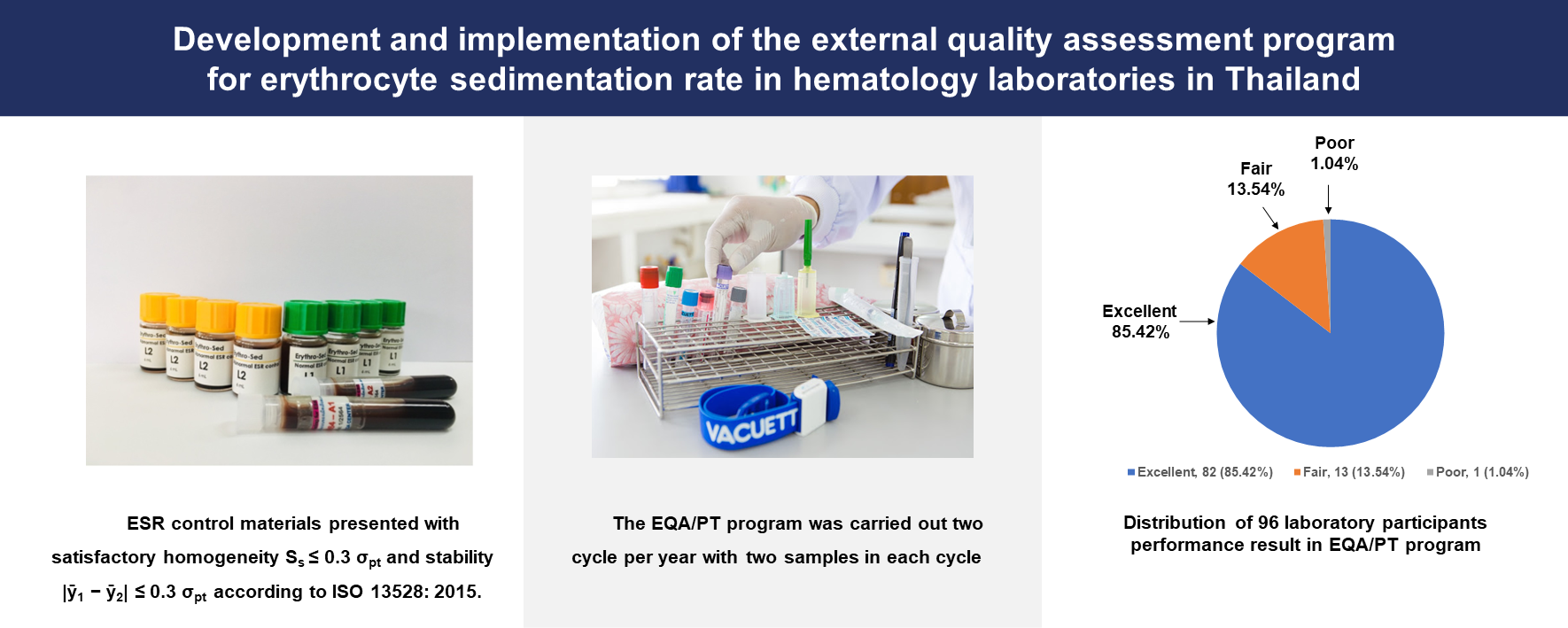
Materials and methods: The PT program for ESR was established at the AMS CMU EQA Unit by the ISO/IEC17043:2010 requirements. The unit produced and sent the ESR control materials to participants for laboratory analysis; the test results were returned to the unit. The PT program was carried out in two cycles per year, with two samples in each cycle. The performance of each laboratory was assessed using robust Z score and performance score.
Results: ESR control materials presented with satisfactory homogeneity and stability according to ISO 13528: 2015. One hundred and four laboratories were enrolled in the PT program. Of these laboratories, 96 (92.3%) returned their data: 40 (42.7%) used the modified Westergren method, 29 (29.2%), 14 (15.7%), and 13 (12.4%) used Microsed, Mixrate, and other methods, respectively. Satisfactory and questionable performance was obtained in 82/96 (85.4.%) and 8/96 (8.3%), respectively. Unsatisfactory performance was noted at 6/96 (6.3%). The level of excellent performance was achieved by 82 (approx. 85%) of these laboratories. Two main types of errors found from analyzing the received data were (i) specimen processing and (ii) incorrect identification of the analytical method.
Conclusion: Our results indicate that the advantage of participation in the PT program for ESR is that the laboratories can monitor and investigate the source of laboratory errors. Therefore, the PT program for ESR testing should become an integral part of the quality assurance system in the laboratory.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Tishkowski K, Gupta V. Erythrocyte Sedimentation Rate. [Updated 2023 Apr 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557485/
Bain BJ. Some influences on the ESR and the fibrinogen level in healthy subjects. Clin Lab Haematol. 1983; 5(1): 45-54. doi: 10.1111/j.1365-2257.1983.tb00495.x.
Alende-Castro V, Alonso-Sampedro M, VazquezTemprano N, Tuñez C, Rey D, García-Iglesias C, Sopeña B, Gude F, Gonzalez-Quintela A. Factors influencing erythrocyte sedimentation rate in adults: New evidence for an old test. Medicine (Baltimore). 2019 Aug; 98(34): e16816. doi: 10.1097/MD.0000000000016816. PMID: 31441853; PMCID: PMC6716712.
Guidelines on selection of laboratory tests for monitoring the acute phase response. International Committee for Standardization in Haematology (expert panel on blood rheology). J clin Pathol. 1988; 41(11):1203-12. doi: 10.1136/jcp.41.11.1203.
Lapić I, Padoan A, Bozzato D, Plebani M. Erythrocyte Sedimentation Rate and C-Reactive Protein in Acute Inflammation: Meta-Analysis of Diagnostic Accuracy Studies. Ame J Clin Pathol. 2020; 153(1): 14-29. doi: 10.1093/ajcp/aqz142.
Baskurt OK, Uyuklu M, Hardeman MR, Meiselman HJ. Photometric measurements of red blood cell aggregation: light transmission versus light reflectance. J Biomed Opt. 2009; 14(5): 54044. doi: 10.1117/1.3251050.
Shelat SG, Chacosky D, Shibutani S. Differences in Erythrocyte Sedimentation Rates Using the Westergren Method and a Centrifugation Method. Am J Clin Pathol. 2008; 130(1): 127-30. doi: 10.1309/E5R9P5YPHXFE3198.
Vennapusa B, de La Cruz L, Shah H, Michalski V, Zhang Q-Y. Erythrocyte Sedimentation Rate (ESR) Measured by the Streck ESR-Auto Plus Is Higher Than With the Sediplast Westergren Method: A Validation Study. Am J Clin Pathol. 2011; 135(3): 386-90. doi: 10.1309/AJCP48YXBDGTGXEV.
Jou JM, Lewis SM, Briggs C, Lee SH, de La Salle B, Mcfadden S. ICSH review of the measurement of the erythocyte sedimentation rate. Int J Lab Hematol. 2011; 33(2): 125-32. doi: 10.1111/j.1751-553X.2011.01302.x.Epub 2011 Feb 25.
Clinical and Laboratory Standards Institute., Jou JM. Procedures for the erythrocyte sedimentation rate test: approved standard. Clinical & Laboratory Standards Institute; 2011. 25.
Herz F, Kaplan E. Effect of Glutaraldehyde Fixation on Erythrocyte Agglutinability. Proc Soc Exp Biol Med. 1973; 144(3): 1017-9. doi: 10.3181/00379727-144-37732.
ISO/IEC 17043 (2010) Conformity assessment-general requirements for proficiency testing. ISO, Geneva.
ISO/IEC 13528 (2015) Statistical methods for use in proficiency testing by interlaboratory comparisons. ISO, Geneva; 2015.
Nilsdotter-Augustinsson A, Briheim G, Herder A, Ljunghusen O, Wahlström O, Ohman L. Inflammatory response in 85 patients with loosened hip prostheses: a prospective study comparing inflammatory markers in patients with aseptic and septic prosthetic loosening. Acta Orthop. 2007; 78(5): 629-39. doi: 10.1080/17453670710014329.
Shaikh KJ, Osio VA, Leeflang MM, Shaikh N. Procalcitonin, C-reactive protein, and erythrocyte sedimentation rate for the diagnosis of acute pyelonephritis in children. Cochrane Database Syst Rev. 2020; 9: CD009185. doi: 10.1002/14651858.CD009185.pub3.
Łój B, Brodowska A, Ciecwiez S, Szydłowska I, Brodowski J, Łokaj M, Starczewski A. The role of serological testing for Chlamydia trachomatis in differential diagnosis of pelvic pain. Ann Agric Environ Med. 2016; 23(3): 506-10. doi: 10.5604/12321966.1219196.
Jones G, Barker A. Reference intervals. Clin Biochem Rev. 2008 Aug;29 Suppl 1(Suppl 1):S93-7. PMCID: PMC2556592.
Lenicek Krleza J, Honovic L, Vlasic Tanaskovic J, Podolar S, Rimac V, Jokic A. Post-analytical laboratory work: national recommendations from the Working Group for Post-analytics on behalf of the Croatian Society of Medical Biochemistry and Laboratory Medicine. Biochem Med. 2019; 29(2): 20502. doi: 10.11613/BM.2019.020502.