AI-based diagnosis of chronic obstructive pulmonary disease from low-dose CT images
Main Article Content
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is a group of diseases characterized by airflow blockage. It is one of the leading causes of global mortality and is primarily attributed to smoking. COPD patients are usually diagnosed by spirometry test. Although regarded as the gold standard for COPD diagnosis, the spirometry test carries contraindications, thus prompting the development of low-dose computed tomography (low-dose CT) scan as an alternative for COPD screening. However, a practical limitation of diagnosing COPD from CT images is its reliance on the expertise of a skilled radiologist.
Objective: To address this limitation, we aimed to develop a deep-learning model for the automated classification of COPD and non-COPD from low-dose CT images.
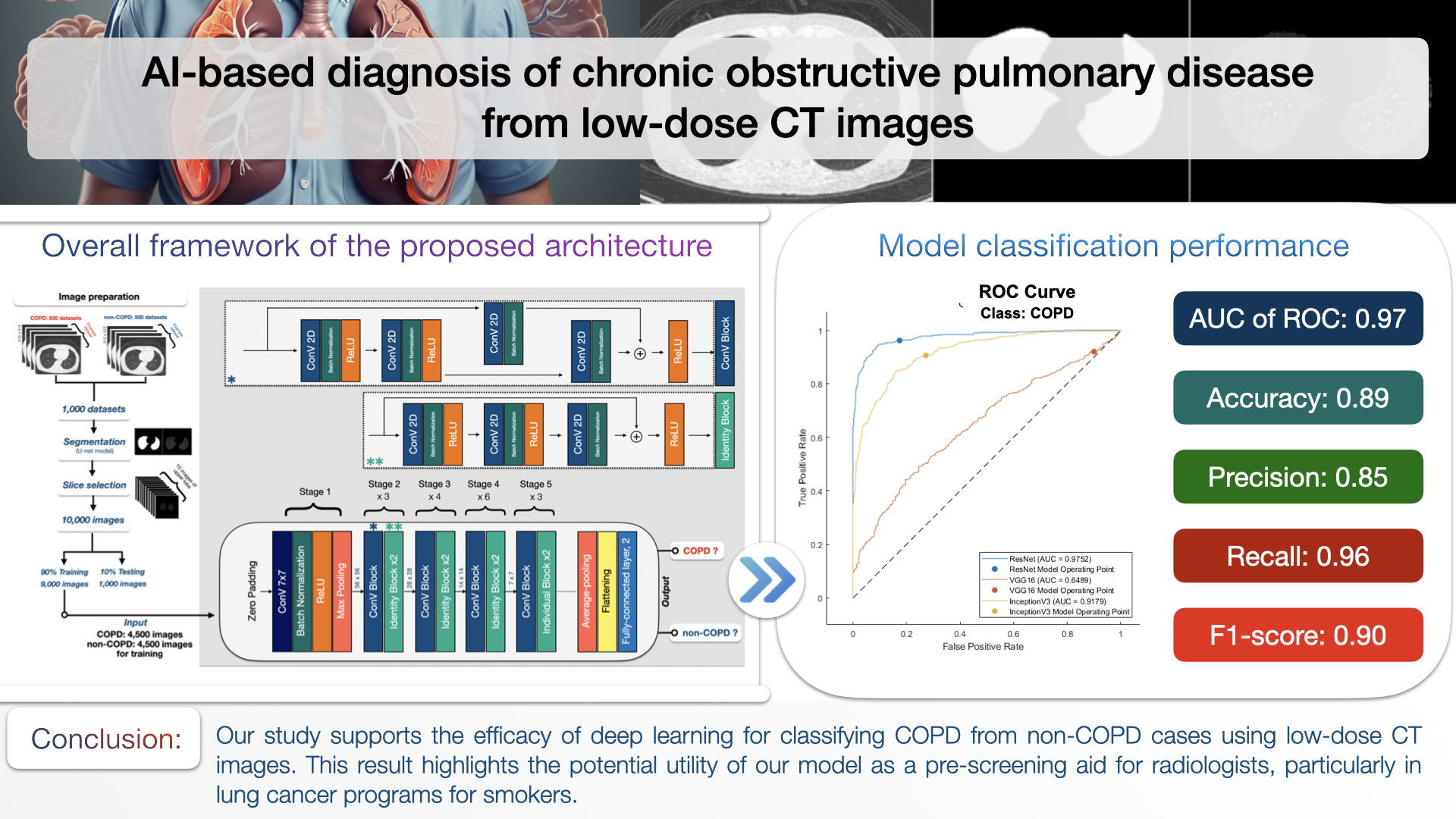
Materials and methods: We examined the potential of a convolutional neural network for identifying COPD. Our dataset consisted of 10,000 low-dose CT images obtained from a lung cancer screening program, involving both ex-smokers and current smokers deemed at high risk of lung cancer. Spirometry data served as the ground truth for defining COPD. We used 90% of the datasets for training and 10% for testing.
Results: Our developed model achieved notable performance metrics: an area under the receiver operating characteristic curve (AUC) of 0.97, an accuracy of 0.89, a precision of 0.85, a recall of 0.96, and an F1-score of 0.90.
Conclusion: Our study demonstrates the potential of deep learning models to augment clinical assessments and improve the diagnosis of COPD, thereby enhancing diagnostic accuracy and efficiency. The findings suggest the feasibility of integrating this technology into routine lung cancer screening programs for COPD detection.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
González G, Ash SY, Vegas-Sánchez-Ferrero G, Onieva JO, Rahaghi FN, Ross JC, et al. Disease staging and prognosis in smokers using deep learning in chest computed tomography. Am J Respir Crit Care Med. 2018; 197(2): 193-203. doi: 10.1164/rccm.201702-0388OC.
MacNee W. Pathology, pathogenesis, and pathophysiology. BMJ. 2006; 332(7551): 1202-4 doi: 10.1136/bmj.332.7551.1202.
Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [Internet]. 2024. Available from: https://goldcopd.org/2024-gold-report/. Accessed February 18, 2024.
Lamprecht B, Soriano JB, Studnicka M, Kaiser B, Vanfleteren LE, Gnatiuc L, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015; 148(4): 971-85. doi: 10.1378/chest.14-2535.
Johns DP, Walters JA, Walters EH, et al. Diagnosis and
early detection of COPD using spirometry. J Thorac Dis. 2014; 6(11): 1557-69. doi: 10.3978/j.issn.20721439.2014.10.11.
Coates AL, Graham BL, McFadden RG. Spirometry in primary care. Can Respir J. 2013; 20(1): 13-22. doi: 10.1155/2013/485906.
Cooper BG. An update on contraindications for lung function testing. Thorax. 2011; 66(8): 714-23 doi: 10.1136/thx.2010.140236.
Gierada DS, Black WC, Chiles C, Pinsky PF, Yankelevitz DF, et al. Low-dose CT screening for lung cancer: Evidence from 2 decades of study. Radiol Imaging Cancer. 2020; 2(2): e190058. doi: 10.1148/rycan.2020190058.
Nawa T. Low-dose CT screening for lung cancer reduced lung cancer mortality in Hitachi City. Int J Radiat Biol. 2019; 95(10): 1441-6. doi: 10.1080/09553002.2019.1614235.
Larke FJ, Kruger RL, Cagnon CH, Flynn M, McNitt-Gray MM, Wu X, et al. Estimated radiation dose associated with low-dose chest CT of average-size participants in the National Lung Screening Trial. AJR Am J Roentgenol. 2011; 197(5): 1165-9. doi: 10.2214/AJR.11.6707.
Bailey KL. The importance of the assessment of pulmonary function in COPD. Med Clin North Am. 2012; 96(4): 745-52. doi: 10.1016/j.mcna.2012.03.009.
Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. Springer International Publishing; 2015. p. 234-41.
Tan W, Huang P, Li X, Ren G, Chen Y, Yang. J. Analysis of segmentation of lung parenchyma based on deep learning methods. J Xray Sci Technol. 2021; 29(6): 945-59. doi: 10.3233/XST-218010.
Nemec SF, Bankier AA, Eisenberg RL. Upper lobepredominant diseases of the lung. AJR Am J Roentgenol. 2013; 200(3): W222-W237. doi: 10.2214/AJR.12.9544.
Tang LYW, Coxson HO, Lam S, Leipsic j, Sin D. Towards large-scale case-finding: Training and validation of residual networks for detection of chronic obstructive pulmonary disease using low-dose CT. Lancet Digit Health. 2020; 2(5): e259–e267. doi: 10.1016/S2589-7500(20)30100-1.
Bradley P, Fuhrman M, Zimmerman M. Pediatric critical care (Fourth Edition). St. Louis; 2011.
He K, Zhang X, Ren S, Sun J, editors. Deep Residual Learning for Image Recognition. 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR); 2016 27-30 June 2016.
Ho TT, Kim T, Kim WJ, Lee CH, chae KJ, Bak SH, et al. A 3D-CNN model with CT-based parametric response mapping for classifying COPD subjects. Sci Rep. 2021; 11: 34. doi: 10.1038/s41598-020-79336-5.
Gierada DS, Bierhals AJ, Choong CK, Bartel ST, Ritter JH, Das NA. et al. Effects of CT section thickness and reconstruction kernel on emphysema quantification relationship to the magnitude of the CT emphysema index. Acad Radiol. 2010; 17(2): 146-56. doi: 10.1016/j.acra.2009.07.025.
Selim M, Zhang J, Fei B, Zhang GQ, Chen J. STAN-CT: Standardizing CT Image using Generative Adversarial Networks. AMIA Annu Symp Proc. 2020; 2020: 1100-9.
Ait Skourt B, El Hassani A, Majda A. Lung CT Image Segmentation Using Deep Neural Networks. Procedia Computer Science. 2018; 127: 109-13.
Murugappan M, Bourisly AK, Prakash NB, Sumithra MG, Acharya UR. et al. Automated semantic lung segmentation in chest CT images using deep neural network. Neural Comput Appl. 2023; 35(21): 15343-64. doi: 10.1007/s00521-022-07399-7.
Rodriguez JD, Perez A, Lozano JA. Sensitivity analysis of k-fold cross validation in prediction error estimation. IEEE Trans Pattern Anal Mach Intell. 2010; 32(3): 569-75. doi: 10.1109/TPAMI.2009.187.
Anguita D, Ghelardoni L, Ghio A, Oneto L, Ridella S. The ‘K’ in K-fold cross validation. In: ESANN 2012 proceedings, European Symposium on Artificial Neural Networks, Computational Intelligence and Machine Learning. Bruges (Belgium), 25-27 April 2012. i6doc. com publ. ISBN 978-2-87419-049-0. Available from: https://www.esann.org/sites/default/files/proceedings/legacy/es2012-62.pdf.