Emerging updates on tracking new landscapes in nanotechnology for the diagnosis and ovarian cancer therapy
Main Article Content
Abstract
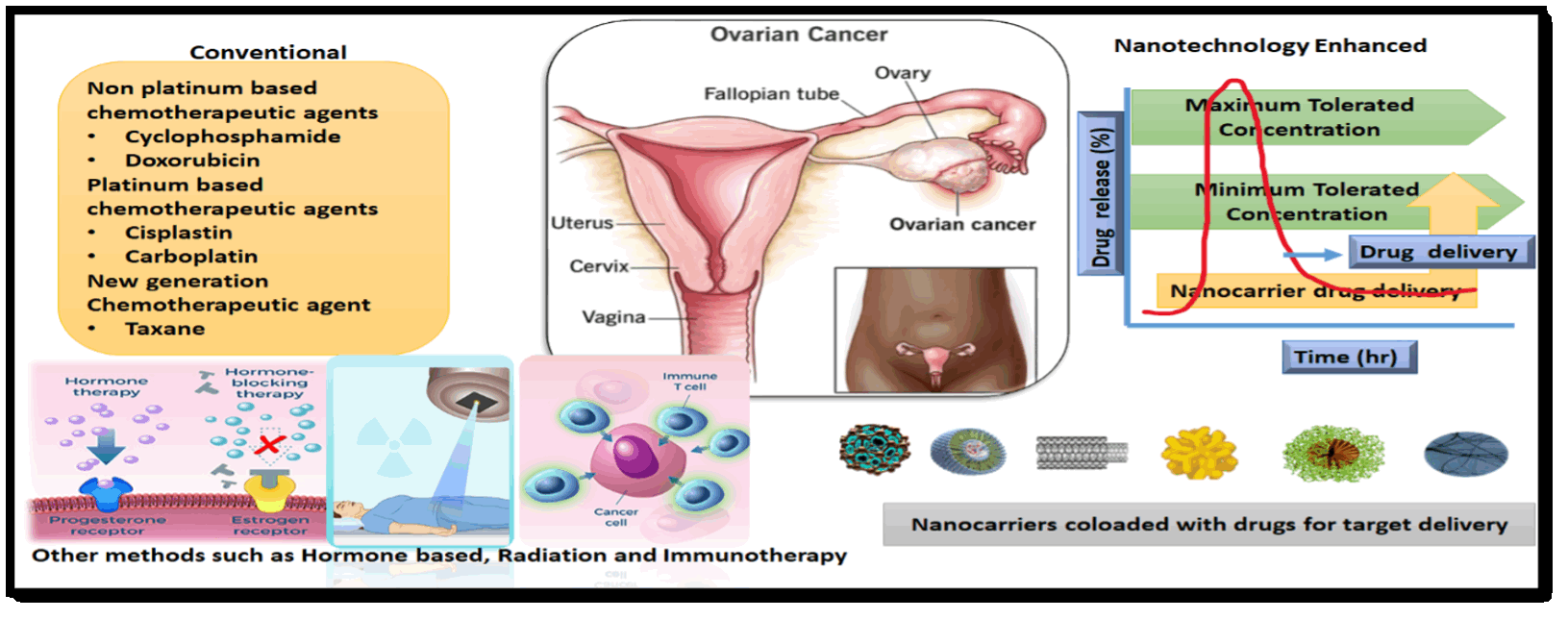
The sixth most common recurrent malignancy worldwide is ovarian cancer in women, and it causes more women to die compared to any other issue impacting the female reproductive system. Ovarian cancer has several histological subgroups differing in clinical traits, risk factors, cell sources, molecular makeups, and treatment possibilities. There is no effective screening procedure, and it is typically discovered at a late stage. Newly found cancer is currently treated with platinum-based chemotherapy and cytoreductive surgery. Due to its recurrence and late diagnosis, ovarian cancer has the highest fatality rates in contrast to all gynecological cancers. The discipline of medical nanotechnology has made great strides in recent years in resolving issues and enhancing the detection and treatment of various illnesses, including cancer. However, most studies and recent reviews on nanotechnology are devoted to how it might be utilized to treat other tumors or disorders. This review’s main objective was the precise diagnosis and treatment of ovarian cancer using nanoscale drug delivery systems. Various nanocarrier systems, such as dendrimers, nanoparticles, liposomes, nanocapsules, and nano micelles, have been discussed. Additionally, we explore how the potency of the combination of immunotherapy and nanotechnology may help to overcome the current therapeutic constraints connected with each application and reveal a novel paradigm in cancer therapy. The unique nanotherapeutic approaches that have demonstrated promising outcomes in preclinical in vivo research are highlighted, along with new nanoformulations actively advancing into clinical trials. Additionally, the possible use of nanomaterials in diagnostic imaging methods and the capacity to use nanotechnology for early ovarian cancer detection are also highlighted.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001; 22(2): 255-88. doi: 10.1210/edrv.22.2.0422.
Zahedi P, Yoganathan R, Piquette-Miller M, Allen C.Recent advances in drug delivery strategies for treatment of ovarian cancer. Expert Opin Drug Deliv. 2012; 9(5): 567-83. doi: 10.1517/17425247.2012.665366.
Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019; 393(10177): 1240-53. doi: 10.1016/S0140-6736(18)32552-2.
Helen HW, Macus C, Kuo T. Improving radiotherapy in cancer treatment: Promises and challenges. Oncotarget. 2017; 8(37): 62742-58. doi: 10.18632/oncotarget.18409.
Khosravi-Shahi P, Cabezón-Gutiérrez L , CustodioCabello S. Metastatic triple negative breast cancer: Optimizing treatment options, new and emerging targeted therapies. Asia Pac J Clin Oncol. 2018; 14(1): 32-9. doi: 10.1111/ajco.12748.
Aggarwal RR, Feng FY, Small EJ. Emerging Categories of Disease in Advanced Prostate Cancer and Their Therapeutic Implications. Oncology (Williston Park). 2017; 31(6): 467-74.
Gao X, Zhang M, Tang Y, Liang X. Cancer cell dormancy: mechanisms and implications of cancer recurrence and metastasis. Onco Targets Ther. 2017; 10: 5219-28.
Baselga NVJ, Hyman DM. A view on drug resistance in cancer. Nature. 2019; 575(7782): 299-309. doi: 10.1038/s41586-019-1730-1.
Rueff J, Rodrigues AS. Cancer Drug Resistance: A Brief Overview from a Genetic Viewpoint. Methods Mol Biol. 2016; 1395: 1-18.doi: 10.1007/978-1-4939-3347-1_1.
Hinohara K, Polyak K. Intratumoral Heterogeneity: More Than Just Mutations, Trends Cell Biol. 2019; 29(7): 569-79. doi: 10.1016/j.tcb.2019.03.003.
Tian Y, Guo R, Wang Y, Yang W. Coordinationinduced assembly of intelligent polysaccharidebased phototherapeutic nanoparticles for cancer treatment. Adv Healthc Mater. 2016; 5 (24): 3099- 104. doi: 10.1002/adhm.201600877.
Yang C, Xia BR, Zhang ZC, et al. Immunotherapy for ovarian cancer: adjuvant, combination, and neoadjuvant. Front Immunol. 2020; 11: 577869. doi: 10.3389/fimmu.2020.577869.
Bozzer S, Dal Bo M, Grimaldi MC, Toffoli G, Macor P. Nanocarriers as a delivery platform for anticancer treatment: biological limits and perspectives in B-cell malignancies. Pharmaceutics. 2022; 14 (9): 1965. doi: 10.3390/pharmaceutics14091965.
Liu Y, Fns M, Lou B, et al. π-π-stacked poly(ɛ- caprolactone)-b-poly(ethylene glycol) micelles loaded with a photosensitizer for photodynamic therapy. Pharmaceutics. 2020; 12(4): 338 doi: 10.3390/pharmaceutics12040338.
Tsouris V, Joo MK, Kim SH, Kwon IC, Won YY. Nano carriers that enable co-delivery of chemotherapy and RNAi agents for treatment of drug-resistant cancers. Biotechnol Adv. 2014; 32(5): 1037-50. doi: 10.1016/j.biotechadv.2014.05.00620.
Shi Y, van der Meel R, Chen X, Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020; 10(17): 7921-4. doi: 10.7150/thno.49577.
Maeda H. Polymer therapeutics and the EPR effect. J Drug Target. 2017; 25(9-10): 781-5. doi: 10.1080/1061186X.2017.1365878.
Rai S, Singh N, Bhattacharya S. Concepts on smart nano-based drug delivery system. Recent Pat Nanotechnol. 2022; 16(1): 67-89. doi: 10.2174/1872210515666210120113738.
Li Y, Gao Y, Zhang X, Guo H, Gao H. Nanoparticles in precision medicine for ovarian cancer: from chemotherapy to immunotherapy. Int J Pharm. 2020; 591: 119986. doi: 10.1016/j.ijpharm.2020.119986.
Wang X, Xiong T, Cui M, et al. A novel targeted codelivery nanosystem for enhanced ovarian cancer treatment via multidrug resistance reversion and mTOR-mediated signaling pathway.J Nanobiotechnol. 2021; 19(1): 444. doi: 10.1186/s12951-021-01139-1.
Barani M, Bilal M, Sabir F, Rahdar A, Kyzas GZ. Nanotechnology in ovarian cancer: diagnosis and treatment. Life Sci. 2021; 266: 118914. doi: 10.1016/j.lfs.2020.118914.
Nishio S, Ushijima K. Clinical significance of primary debulking surgery and neoadjuvant chemotherapyinterval debulking surgery in advanced ovarian cancer. Jpn J Clin Oncol. 2020; 50(4): 379-86. doi: 10.1093/jjco/hyaa015.
Prat J. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014; 124 (1): 1-5. doi: 10.1016/j.ijgo.2013.10.001.
Kim S, Han Y, Kim SI, Kim HS, Kim SJ, Song YS. Tumor evolution and chemoresistance in ovarian cancer. NPJ Precis Oncol. 2018; 2: 20. doi: 10.1038/s41698-018-0063-0. eCollection 2018.
Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006; 95 (Suppl. S1), S161-S192. doi: 10.1016/S0020-7292(06)60033-7.
Salani R, Axtell A, Gerardi M, Holschneider C, Bristow RE. Limited utility of conventional criteria for predicting unresectable disease in patients with advanced stage epithelial ovarian cancer. Gynecol Oncol. 2008; 108(2):271-5. doi: 10.1016/j.ygyno.2007.11.004.
Schorge JO, McCann C, Del Carmen MG. Surgical debulking of ovarian cancer: What difference does it make? Rev Obstet Gynecol. 2010; 3(3): 111-7.
Du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGOOVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009; 115(6): 1234-44. doi: 10.1002/cncr.24149.
Basta ABM, Bie ´nkiewicz A, Blecharz P, Bodnar L, Jach R, Knapp P, et al. Recommendation of the Polish society of oncological gynaecology for the diagnosis and treatment of ovarian cancer. Oncol Clin Pract. 2017; 15 (1): 5-23. doi: 10.15557/CGO.2017.0001.
Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen, et al. Paclitaxel plus platinumbased chemotherapy versus conventional platinumbased chemotherapy in women with relapsed ovarian cancer: The ICON4/AGO-OVAR-2.2 trial. Lancet. 2003; 361(9375): 2099-106. doi: 10.1016/s0140-6736(03)13718-x.
Ozaki S, Takigawa N, Ichihara E, Hotta K, Oze I, Kurimoto E, et al. Favorable response of heavily treated Wilms’ tumor to paclitaxel and carboplatin. Onkologie. 2012; 35(5): 283-6. doi: 10.1159/000338532.
Cristea M, Han E, Salmon L, Morgan RJ. Practical considerations in ovarian cancer chemotherapy. Ther Adv Med Oncol. 2010; 2(3): 175-87. doi: 10.1177/1758834010361333.
Högberg T, Glimelius B, Nygren P. A systematic overview of chemotherapy effects in ovarian cancer. Acta Oncol. 2001; 40(2-3): 340-60. doi: 10.1080/02841860151116420.
Aabo K, Adams M, Adnitt P, Alberts DS, Athanazziou A, Barley V, et al. Chemotherapy in advanced ovarian cancer: Four systematic meta-analyses of individual patient data from 37 randomized trials. Advanced Ovarian Cancer Trialists’ Group. Br J Cancer. 1998; 78(11): 1479-87. doi: 10.1038/bjc.1998.710.
Neijt JP, Engelholm SA, Tuxen MK, Sorensen PG, Hansen M, Sessa C, et al. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J Clin Oncol. 2000; 18(17): 3084-92. doi: 10.1200/JCO.2000.18.17.3084.
Pokhriyal R, Hariprasad R, Kumar, L, Hariprasad G. Chemotherapy resistance in advanced ovarian cancer patients. Biomark Cancer. 2019; 11: 1179299X19 860815. doi: 10.1177/1179299X19860815.
Damia G, Broggini M. Platinum Resistance in Ovarian Cancer: Role of DNA Repair. Cancers. 2019; 11(1): 119. doi: 10.3390/cancers11010119.
Davis A, Tinker AV, Friedlander M. “Platinum resistant” ovarian cancer: What is it, who to treat and how to measure benefit? Gynecol Oncol. 2014; 133(3): 624- 31. doi: 10.1016/j.ygyno.2014.02.038.
Li H, Liu ZY, Wu N, Chen YC, Cheng Q, Wang J. PARP inhibitor resistance: The underlying mechanisms and clinical implications. Mol Cancer. 2020; 19(1): 107. doi: 10.1186/s12943-020-01227-0.
Haley B, Frenkel E: Nanoparticles for drug delivery in cancer treatment. Urol Oncol. 2008; 26(1): 57-64. doi: 10.1016/j.urolonc.2007.03.015.
Macchione MA, Biglione C, Strumia M. Design, synthesis and architectures of hybrid nanomaterials for therapy and diagnosis applications. Polymers. 2018; 10(5): 527. doi.org/10.3390/polym10050527.
Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018; 3: 7. doi.org/10.1038/s41392-017-0004-3.
Desai KGH. Polymeric drug delivery systems for intraoral site-specific chemoprevention of oral cancer. J Biomed Mater Res. 2018; Part B, 106(3): 1383-413. doi: 10.1002/jbm.b.33943. Epub 2017 Jun 26.
Hekman MCH, Boerman OC, Bos DL, Massuger LFAG, Weil S, Grasso L, et al. Improved intraoperative detection of ovarian cancer by folate receptor alpha targeted dual-modality imaging. Mol Pharm. 2017; 14: 3457-63.
Judy RP, Keating JJ, DeJesus EM, Jiang JX, Okusanya OT, Nie S, et al Quantification of tumor fluorescence during intraoperative optical cancer imaging. Sci Rep. 2015; 5: 16208. doi: 10.1038/srep16208.
Falagan-Lotsch P, Grzincic EM, Murphy CJ.. New Advances in Nanotechnology-Based Diagnosis and Therapeutics for Breast Cancer: An Assessment of Active-Targeting Inorganic Nanoplatforms. Bioconjug Chem. 2017; 18; 28(1): 135-52. doi: 10.1021/acs.bioconjchem.6b00591.
Giulio Caraccioloa, Hojatollah Vali b, Anna Moorec, Morteza Mahmoudi. Challenges in molecular diagnostic research in cancer nanotechnology, Nano Today. 2019; 27: 6-10. doi.org/10.1016/j.nantod.2019.06.001.
www.who.int/gho/publications/worldhealthstatistics/2017.
M, Lippi G. Cancer diagnostics: current concepts and future perspectives. Ann Transl Med. 2017; 5(13): 268. doi: 10.21037/atm.2017.06.20.
Caracciolo G, Safavi-Sohi R, Malekzadeh R, Poustchi H, Vasighi M, Chiozzi RZ, et al. Nanoscale Horizons Correction: Disease-specific protein corona sensor arrays may have disease detection capacity nanoscale. Horiz. 2018; 5: 372. doi.org/10.1039/c9nh00097f.
Smith B.R., Gambhir S.S., Engineered ManganeseBased Nanoparticles as MRI Contrast Agents for Early Tumor Detection. Chem Rev. 2017; 117(3): 901-86.
Duvshani-Eshet M, Benny O, Morgenstern A, Machluf M. Therapeutic ultrasound facilitates antiangiogenic gene delivery and inhibits prostate tumor growth. Mol Cancer Ther. 2007; 6(8):2371- 82. doi: 10.1158/1535-7163.MCT-07-0019.
. Deng Z, Yan F, Jin Q, Li F, Wu J, Liu X, et al. Reversal of multidrug resistance phenotype in human breast cancer cells using doxorubicin-liposomemicrobubble complexes assisted by ultrasound. J Control Release. 2014; 174, 109-16. doi: 10.1016/j.jconrel.2013.11.018.
Bisi A, Cappadone C, Rampa A, Farruggia G, Sargenti A, Belluti F, et al. Coumarin derivatives as potential antitumor agents: Growth inhibition, apoptosis induction and multidrug resistance reverting activity. Eur J Med Chem. 2017; 127: 577-85. doi: 10.1016/j.ejmech.2017.01.020.
Mignani S, Rodrigues J, Thomas H, Zablocka M, Shi X, Caminade AM, et al. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem Soc Rev. 2018; 47: 514-32.
Liu X, Dong J, Cai W, Pan Y, Li,R, Li B. The effect of thymoquinone on apoptosis of SK-OV-3 ovarian cancer cell by regulation of Bcl-2 and bax. Int. J. Gynecol Cancer. 2017; 27(8): 1596-601. doi: 10.1097/ IGC.0000000000001064.
Qiu M, Xue C, Zhang L. Piperine alkaloid induces anticancer and apoptotic effects in cisplatin resistant ovarian carcinoma by inducing G2/M phase cell cycle arrest, caspase activation and inhibition of cell migration and PI3K/Akt/GSK3 signaling pathway. J Balkan Union Oncol. 2019; 24: 2316-21.
Ren MX, Deng XH, Ai F, Yuan GY, Song HY. Effect of quercetin on the proliferation of the human ovarian cancer cell line SKOV-3 in vitro. Exp Ther Med. 2015; 10(2): 579-83. doi: 10.3892/etm.2015.2536.
Xu X, Shi J, Gao H, Li Q. Zeylenone inhibits proliferation and promotes apoptosis in ovarian carcinoma cells via Janus kinase 2/signal transducers and activators of transcription 3 pathways. J Obstet Gynaecol Res. 2018; 44(8): 1451-7. doi: 10.1111/jog.13690.
Pourhanifeh MH, Darvish M, Tabatabaeian J, Fard MR, Mottaghi R, Azadchehr MJ, Jahanshahi M, et al. Therapeutic role of curcumin and its novel formulations in gynecological cancers. J Ovarian Res. 2020; 13: 130. doi: 10.1186/s13048-020-00731-7.
Teekaraman D, Elayapillai SP, Viswanathan MP, Jagadeesan A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA- 1cell line. Chem Biol Interact. 2019; 300: 91-100. doi: 10.1016/j.cbi.2019.01.008.
Park S, Lim W, Jeong W, Bazer FW, Lee D, Song G. Sideroxylin (Callistemon lanceolatus) suppressed cell proliferation and increased apoptosis in ovarian cancer cells accompanied by mitochondrial dysfunction, the generation of reactive oxygen species, and an increase of lipid peroxidation. J Cell Physiol. 2018; 233(11): 8597-604. doi: 10.1002/ jcp.26540.
Gao X, Wang B, Wei X, Men K, Zheng F, Zhou Y, et al. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale. 2012; 4(22): 7021-30. doi: 10.1039/c2nr32181e.
Ryman-Rasmussen, Jessica P, et al. “Penetration of Intact Skin by Quantum Dots with Diverse Physicochemical Properties.” Toxicol Sci. 2006; 91(1): 159-65 doi: 10.1093/toxsci/kfj122.
Jia G, Han Y, An Y, Ding Y, He C, Wang X, et al . NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018; 178, 302-16. doi.org/10.1016/j.biomaterials.2018.06.029.
Jiang J, Oberdörster, G, Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res. 2009; 11: 77-89. doi.org/10.1007/s11051-008-9446-4.
Awasthi R, Pant I, T Kulkarni G, Satiko Kikuchi I, de Jesus Andreoli Pint T, et al. Opportunities and challenges in nanostructure mediated drug delivery: Where do we stand?. Curr Nanomed. 2016; 6(2): 78- 104. doi: 10.2174/2468187306666160808160330.
Dobrovolskaia MA, Aggarwa P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharmaceutics. 2008; 5(4): 487-95. doi: 10.1021/mp800032f.
Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008: 26(5), 561-9. doi.org/10.1038/nbt1402.
Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proceedings of the National Academy of Sciences of the United States of America. 2010; 107(5): 1864-9. doi.org/10.1073/pnas.0910603106.
Tansathien K, Dechsri K, Opanasopit P, Nuntharatanapong N, Sukma M, Rangsimawong W. Investigational lipid nanocarriers and microspicule gel for dermal delivery of porcine placenta extract. J Curr Sci Technol. 2022; 12(3): 505-16. doi: 10.14456/jcst.2022.39.
Thungsatianpun N, Mavichak R, T-Thienprasert N, Unajak S, Sinthuvanich C. Cell-penetrating peptide nanocomplexes enhanced cellular uptake of dsRNA in Sf9 cell line. J Curr Sci Technol. 2021; 11(2): 299- 310. doi: 10.14456/jcst.2021.30.
Chamsai B, Opanasopit P, Samprasit W. Dual-drugsloaded polymeric nanoparticles formulation design based on response surface methodology of particle size and zeta potential. J Curr Sci Technol. 2020; 10 (2): 143-53.