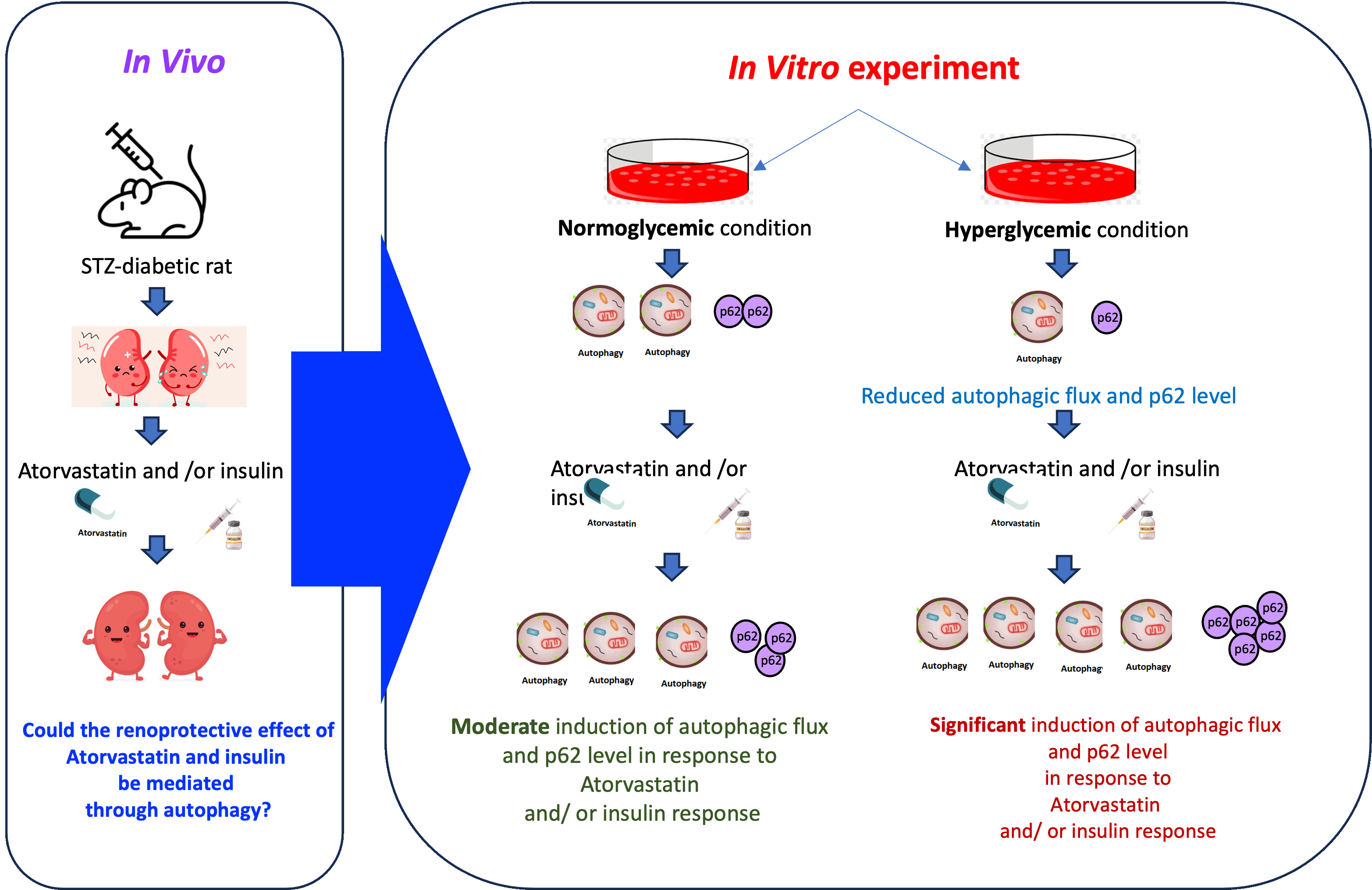
Atorvastatin increases autophagic flux and p62/SQSTM1 of kidney cells in hyperglycemic conditions and treatment in combination with insulin improves renal function of streptozotocin (STZ)-induced diabetic rats
Main Article Content
Abstract
Background: Although atorvastatin is commonly used as a hypolipidemic agent, it confers many health benefits in which the underlying mechanisms are not fully understood. We have previously shown that combined treatment of atorvastatin and insulin effectively restored renal function of streptozotocin (STZ)-induced diabetic rats; nevertheless, the underlying mechanism was not known.
Objective: To determine whether the reno-protective effect of atorvastatin and insulin is mediated through its impact on autophagy.
Materials and methods: Markers of autophagy, LC3, and p62/SQSTM1, in rat kidney tissues and cell lines treated with atorvastatin and/or insulin were determined by Western blot analysis.
Results: Levels of both LC3-I and LC3-II proteins in kidney tissues of STZ-diabetic rats treated with atorvastatin and insulin were significantly increased. The autophagic flux was examined in vitro and showed that high glucose culture conditions suppressed the autophagic flux in kidney cells. Treatment with insulin moderately increased the conversion of LC3-I to LC3-II. Interestingly, atorvastatin increased autophagic flux only in the hyperglycemic but not in the normoglycemic condition. p62/SQSTM1 protein level was decreased in response to high glucose treatment but increased with the addition of insulin and/or atorvastatin.
Conclusion: This study has demonstrated that atorvastatin may represent a novel regimen in providing prevention and protection for diabetic nephropathy through the underlying mechanisms of inducing autophagy and p62/SQSTM1.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Hovind P, Tarnow L, Rossing K, Rossing P, Eising S, Larsen N, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003; 26(4): 1258-64. doi: 10.2337/diacare.26.4.1258.
Ritz E, Keller C, Bergis K, Strojek K. Pathogenesis and course of renal disease in IDDM/NIDDM: differences and similarities. Am J Hypertens. 1997; 10(9 Pt 2): 202S-7S. doi: 10.1016/s0895-7061(97)00154-4.
Burdo JR, Chen Q, Calcutt NA, Schubert D. The pathological interaction between diabetes and presymptomatic Alzheimer’s disease. Neurobiol Aging. 2009; 30(12): 1910-7. doi: 10.1016/j.neurobiolaging.2008.02.010.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010; 107(9): 1058-70. doi: 10.1161/CIRCRESAHA.110.223545.
Ruggenenti P, Remuzzi G. What blood-pressure level provides greatest renoprotection in patients with diabetic nephropathy and hypertension? Nat Clin Pract Nephrol. 2006; 2(5): 250-1. doi: 10.1038/ncpneph0157.
Yamamoto T, Takabatake Y, Kimura T, Takahashi A, Namba T, Matsuda J, et al. Time-dependent dysregulation of autophagy: Implications in aging and mitochondrial homeostasis in the kidney proximal tubule. Autophagy. 2016; 12(5): 801-13. doi: 10.1080/15548627.2016.1159376.
Jiang Y, Zhao Y, Zhu X, Liu Y, Wu B, Guo Y, et al. Effects of autophagy on macrophage adhesion and migration in diabetic nephropathy. Ren Fail. 2019; 41(1): 682- 90. doi: 10.1080/0886022X.2019.1632209.
Liu WJ, Huang WF, Ye L, Chen RH, Yang C, Wu HL, et al. The activity and role of autophagy in the pathogenesis of diabetic nephropathy. Eur Rev Med Pharmacol Sci. 2018; 22(10): 3182-9. doi: 10.26355/eurrev_201805_15079.
Xu Y, Liu L, Xin W, Zhao X, Chen L, Zhen J, et al. The renoprotective role of autophagy activation in proximal tubular epithelial cells in diabetic nephropathy. J. Diabetes Complicat. 2015; 29(8): 976-83. doi: 10.1016/j.jdiacomp.2015.07.021.
Thongnak L, Pongchaidecha A, Jaikumkao K, Chatsudthipong V, Chattipakorn N, Lungkaphin A. The additive effects of atorvastatin and insulin on renal function and renal organic anion transporter 3 function in diabetic rats. Sci Rep. 2017; 7(1): 13532. doi: 10.1038/s41598-017-13206-5.
Iadnut A, Mamoon K, Thammasit P, Pawichai S, Tima S, Preechasuth K, et al. In Vitro Antifungal and Antivirulence Activities of Biologically Synthesized Ethanolic Extract of Propolis-Loaded PLGA Nanoparticles against Candida albicans. Evid Based Complement Alternat Med. 2019; 2019: 3715481. doi: 10.1155/ 2019/3715481.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9(7): 671-5. doi: 10.1038/nmeth.2089.
Tanida I, Ueno T, Kominami E. LC3 and Autophagy. Methods Mol Biol. 2008; 445: 77-88. doi: 10.1007/978-1-59745-157-4_4.
Cui J, Bai X, Chen X. Autophagy and Diabetic Nephropathy. Adv Exp Med Biol. 2020; 1207: 487-94. doi: 10.1007/978-981-15-4272-5_36.
Koch EAT, Nakhoul R, Nakhoul F, Nakhoul N. Autophagy in diabetic nephropathy: a review. Int Urol Nephrol. 2020; 52(9): 1705-12. doi: 10.1007/s11255-020-02545-4.
Zhang MZ, Wang Y, Paueksakon P, Harris RC. Epidermal growth factor receptor inhibition slows progression of diabetic nephropathy in association with a decrease in endoplasmic reticulum stress and an increase in autophagy. Diabetes. 2014; 63(6): 2063-72. doi: 10.2337/db13-1279.
Kobayashi S, Xu X, Chen K, Liang Q. Suppression of autophagy is protective in high glucose-induced cardiomyocyte injury. Autophagy. 2012; 8(4): 577-92. doi: 10.4161/auto.18980.
Bhatia D, Choi ME. Autophagy in kidney disease: Advances and therapeutic potential. Prog Mol Biol Transl Sci. 2020; 172: 107-33. doi: 10.1016/bs.pmbts.2020.01.008.
Han K, Zhou H, Pfeifer U. Inhibition and restimulation by insulin of cellular autophagy in distal tubular cells of the kidney in early diabetic rats. Kidney Blood Press Res. 1997; 20(4): 258-63. doi: 10.1159/000174155.
Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011; 12(1): 21-35. doi: 10.1038/nrm3025.
Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010; 40(2): 310-22. doi: 10.1016/j.molcel.2010.09.026.
Sabe AA, Elmadhun NY, Dalal RS, Robich MP, Sellke FW. Resveratrol regulates autophagy signaling in chronically ischemic myocardium. J Thorac Cardiovasc Surg. 2014; 147(2): 792-8; Discussion 8-9. doi: 10.1016/j.jtcvs.2013.06.062.
Zhang Q, Yang YJ, Wang H, Dong QT, Wang TJ, Qian HY, et al. Autophagy activation: a novel mechanism of atorvastatin to protect mesenchymal stem cells from hypoxia and serum deprivation via AMP-activated protein kinase/mammalian target of rapamycin pathway. Stem Cells Dev. 2012; 21(8): 1321-32. doi: 10.1089/scd.2011.0684.
Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Seo YH, et al. Additive beneficial effects of fenofibrate combined with atorvastatin in the treatment of combined hyperlipidemia. J Am Coll Cardiol. 2005; 45(10): 1649-53. doi: 10.1016/j.jacc.2005.02.052.
Yang F, Wang J, Li F, Cui L. Atorvastatin Combined Nitroglycerin Therapy Confer Additive Effects on Rabbits with Dyslipidemia. Exp Clin Endocrinol Diabetes. 2016; 124(6): 367-71. doi: 10.1055/s-0042-104496.
Koh KK, Quon MJ, Han SH, Lee Y, Park JB, Kim SJ, et al. Additive beneficial effects of atorvastatin combined with amlodipine in patients with mild-to-moderate hypertension. Int J Cardiol. 2011; 146(3): 319-25. doi: 10.1016/j.ijcard.2009.07.002.
Lu JC, Cui W, Zhang HL, Liu F, Han M, Liu DM, et al. Additive beneficial effects of amlodipine and atorvastatin in reversing advanced cardiac hypertrophy in elderly spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2009; 36(11): 1110-9. doi: 10.1111/j.1440-1681.2009.05198.x.
Ke X, Ke B, Wang X, Wu S, Yang R, Hu C. Additive effects of atorvastatin combined with sitagliptin on rats with myocardial infarction: a pilot study. Arch Med Sci. 2017;1 3(4): 956-61. doi: 10.5114/aoms.2017.68143.
Elmadhun NY, Lassaletta AD, Chu LM, Liu Y, Feng J, Sellke FW. Atorvastatin increases oxidative stress and modulates angiogenesis in Ossabaw swine with the metabolic syndrome. J Thorac Cardiovasc Surg. 2012; 144(6): 1486-93. doi: 10.1016/j.jtcvs.2012.08.065.
Wei YM, Li X, Xu M, Abais JM, Chen Y, Riebling CR, et al. Enhancement of autophagy by simvastatin through inhibition of Rac1-mTOR signaling pathway in coronary arterial myocytes. Cell Physiol Biochem. 2013; 31(6): 925-37. doi: 10.1159/000350111.
Gao K, Wang G, Wang Y, Han D, Bi J, Yuan Y, et al. Neuroprotective Effect of Simvastatin via Inducing the Autophagy on Spinal Cord Injury in the Rat Model. Biomed Res Int. 2015; 2015: 260161. doi: 10.1155/2015/260161.
Sabe AA, Elmadhun NY, Sadek AA, Chu LM, Bianchi C, Sellke FW. Differential effects of atorvastatin on autophagy in ischemic and nonischemic myocardium in Ossabaw swine with metabolic syndrome. J Thorac Cardiovasc Surg. 2014; 148(6): 3172-8. doi: 10.1016/j.jtcvs.2014.07.104.
Song YM, Song SO, Jung YK, Kang ES, Cha BS, Lee HC, et al. Dimethyl sulfoxide reduces hepatocellular lipid accumulation through autophagy induction. Autophagy. 2012; 8(7): 1085-97. doi: 10.4161/auto.20260.
Riley BE, Kaiser SE, Kopito RR. Autophagy inhibition engages Nrf2-p62 Ub-associated signaling. Autophagy. 2011; 7(3): 338-40. doi: 10.4161/auto.7.3.14780.
Sha Z, Schnell HM, Ruoff K, Goldberg A. Rapid induction of p62 and GABARAPL1 upon proteasome inhibition promotes survival before autophagy activation. J Cell Biol. 2018; 217(5): 1757-76. doi: 10.1083/jcb.201708168.
Puissant A, Fenouille N, Auberger P. When autophagy meets cancer through p62/SQSTM1. Am J Cancer Res. 2012; 2: 397-412.
Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1982; 79(6): 1889-92. doi: 10.1073/pnas.79.6.1889.
Paine MG, Babu JR, Seibenhener ML, Wooten MW. Evidence for p62 aggregate formation: role in cell survival. FEBS Lett. 2005; 579(22): 5029-34. doi: 10.1016/j.febslet.2005.08.010.
Gal J, Strom AL, Kilty R, Zhang F, Zhu H. p62 accumulates and enhances aggregate formation in model systems of familial amyotrophic lateral sclerosis. J Biol Chem. 2007; 282(15): 11068-77. doi: 10.1074/jbc.M608787200.
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005; 171(4): 603-14. doi: 10.1083/jcb.200507002.
Su H, Wang X. Autophagy and p62 in cardiac protein quality control. Autophagy. 2011; 7(11): 1382-3. doi: 10.4161/auto.7.11.17339.