Correlation between high-sensitivity cardiac troponin I, lactate levels, and clinical outcomes in on-pump coronary artery bypass grafting
Main Article Content
Abstract
Background: On-pump coronary artery bypass graft (CABG) causes myocardial damage and hypoperfusion. However, it is unknown how varied timings of combining serum high-sensitivity cardiac troponin I (hs-cTnI) and lactate levels in on-pump CABG surgery would affect clinical outcomes.
Objectives: This study aims to evaluate serum hs-cTnI and lactate levels and their influence on postoperative clinical outcomes in patients with on-pump CABG.
Materials and methods: Eleven coronary artery disease (CAD) patients were included for on-pump CABG surgery. The biomarkers were collected at four stages of onpump CABG: before sternotomy (T0, pre-cardiopulmonary bypass (pre-CPB)), 5 minutes before aortic cross-clamp of CPB (T1, preaortic cross-clamp), after aortic cross-clamp (T2, postaortic cross-clamp), and 24 hrs post-surgery in an intensive care unit (T3, ICU at 24 hrs).
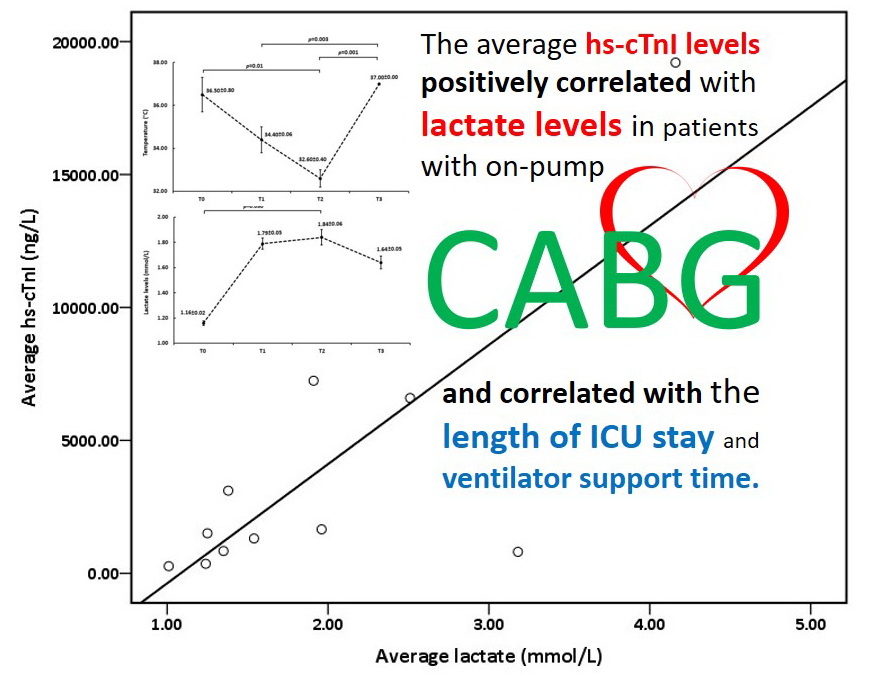
Results: Correlation analysis revealed that during the study period, average hs-cTnI is positively associated with lactate levels (r=0.775, p=0.005). However, 24 hours after surgery, lactate levels return more quickly than hs-cTnI levels. The average hs-cTnI and lactate levels were positively correlated with CPB time and aortic clamp time. Regarding clinical outcomes, average hs-cTnI, and lactate levels were positively associated with a length of ICU stay (r=0.717 and 0.612, p=0.013 and 0.045, respectively). However, only the lactate levels were associated with ventilator support time (r=0.674, p=0.023).
Conclusion: We demonstrated that hs-cTnI and lactate levels are important markers of myocardial injury in association with hypoperfusion during on-pump CABG, and it could be used to monitor the postoperative outcome.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Diodato M, Chedrawy EG. Coronary artery bypass graft surgery: the past, present, and future of myocardial revascularisation. Surg Res Pract. 2014; 1: 1-6. doi: 10.1155/2014/726158.
Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005; 111(25): 3481-8. doi.org/10.1161/CIRCULATIONAHA.105.537878.
Hawkes AL, Nowak M, Bidstrup B, Speare R. Outcomes of coronary artery bypass graft surgery. Vasc Health Risk Manag. 2006; 2(4): 477-84. doi: 10.2147/vhrm.2006.2.4.477.
De Hert S, Moerman A. Myocardial injury and protection related to cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol. 2015; 29(2): 137-49. doi: 10.1016/j.bpa.2015.03.002.
Ferguson TB. Ischemia/reperfusion injury in coronary artery bypass grafting: Time to revisit? J Thorac Cardiovasc Surg. 2011; 141(1): 1-2. doi: 10.1016/j. jtcvs.2010.04.008.
Naik R, George G, Sathappan K. Hyperlactatemia in patients undergoing adult cardiac surgery under cardiopulmonary bypass: Causative factors and its effect on surgical outcome. Ann Card Anaesth. 2016; 19(4): 668-75. doi: 10.4103/0971-9784.191579.
Kushimoto S, Akaishi S, Sato T, et al. Lactate, a useful marker for disease mortality and severity but an unreliable marker of tissue hypoxia/hypoperfusion in critically ill patients. Acute Med Surg. 2016; 3(4): 293-7. doi: 10.1002/ams2.207.
Stephens EH, Epting CL, Backer CL, Wald EL. Hyperlactatemia: an update on postoperative lactate. World J Pediatr Congenit Heart Surg. 2020; 11(3): 316- 24. doi: 10.1177/215013512090397.
Scolari FL, Schneider D, Fogazzi DV, Gus M, Rover MM, Bonatto MG, et al. Association between serum lactate levels and mortality in patients with cardiogenic shock receiving mechanical circulatory support: a multicenter retrospective cohort study. BMC Cardiovasc Disord. 2020; 20(1): 1-10. doi: 10.1186/s12872-020-01785-7.
Ryoo SM, Kim WY. Clinical applications of lactate testing in patients with sepsis and septic shock. J Emerg Crit Care Med. 2018; 2(2): 14. doi: 10.21037/jeccm.2018.01.13.
Ooi DS, Isotalo PA, Veinot JP. Correlation of antemortem serum creatine kinase, creatine kinase-MB, troponin I, and troponin T with cardiac pathology. Clin Chem. 2000; 46(3): 338-44. doi: 10.1093/clinchem/46.3.338.
Babuin L, Jaffe AS. Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ. 2005; 173(10): 1191-202. doi: 10.1503/cmaj/051291.
Schmid J, Liesinger L, Birner-Gruenberger R, Stojakovic T, Scharnagl H, Dieplinger B, et al. Elevated cardiac troponin T in patients with skeletal myopathies. J Am Coll Cardiol. 2018; 71(14): 1540-9. doi: 10.1016/j. jacc.2018.01.070.
Sharma S, Jackson P, Makan J. Cardiac troponins. BMJ Publishing Group; 2004. p. 1025-6. doi: 10.1136/ jcp.2003.015420.
Garg P, Morris P, Fazlanie AL, Vijayan S, Dancso B, Dastidar AG, et al. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern Emerg Med. 2017; 12: 147-55. doi: 10.1007/s11739-017-1612-1.
Shah AS, Anand A, Strachan FE, et al. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, clusterrandomised controlled trial. Lancet. 2018; 392: 919-28. doi: 10.1016/S0140-6736(18)31923-8.
O’Carroll-Kuehn BU, Meeran H. Management of coagulation during cardiopulmonary bypass. Cont Educ Anaesth Crit Care Pain. 2007(6); 7: 195-8. doi.org/10.1093/bjaceaccp/mkm03.
Wahba A, Milojevic M, Boer C, et al. 2019 EACTS/ EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Eur J Cardiothorac Surg. 2020; 57(2): 210-51. doi: 10.1093/ejcts/ezz267.
Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/ EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019; 40: 87-165. doi.org/10.1093/eurheartj/ehy394.
Greenson N, Macoviak J, Krishnaswamy P,et al. Usefulness of cardiac troponin I in patients undergoing open heart surgery. Am Heart J. 2001; 141(3): 447-55. doi: 10.1067/mhj.2001.113071.
Domanski MJ, Mahaffey K, Hasselblad V, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. Jama. 2011; 305(6): 585-91. doi: 10.1001/jama.2011.99.
Thygesen K, Alper JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018; 72: 2231-64. doi: 10.1016/j. jacc.2018.08.1038.
Piper HM, Meuter K, Schäfer C. Cellular mechanisms of ischemia-reperfusion injury. Ann Thorac Surg. 2013; 75(2): S644-8. doi: 10.1016/s0003-4975(02)04686-6.
Januzzi Jr JL. Troponin testing after cardiac surgery. 2009; 1(3): 22-32.
Chauin A. The Main Causes and Mechanisms of Increase in Cardiac Troponin Concentrations Other Than Acute Myocardial Infarction (Part 1): Physical Exertion, Inflammatory Heart Disease, Pulmonary Embolism, Renal Failure, Sepsis. Vasc Health Risk Manag. 2021; 17: 601-17. doi: 10.2147/VHRM.S327661.
Bakker J, Coffernils M, Leon M, et al. Blood lactate levels are superior to oxygen-derived variables in predicting outcome in human septic shock. Chest. 1991; 99(4): 956-62. doi: 10.1378/chest.99.4.956.
Lee YS, Kim WY, Yoo JW, et al. Correlation between regional tissue perfusion saturation and lactate level during cardiopulmonary bypass. Korean J Anesthesiol. 2018; 71(5): 361-7. doi: 10.4097/kja.d.17.00002.
Mitchell SC, Vinnakota A, Deo SV, et al. Relationship between intraoperative serum lactate and hemoglobin levels on postoperative renal function in patients undergoing elective cardiac surgery. J Card Surg. 2018; 33(6): 316-21. doi: 10.1111/jocs.13713.
Wahba A, Milojevic M, Boer C, et al. 2019 EACTS/ EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Eur J Cardiothorac Surg. 2020; 57(2): 210-51. doi: 10.1093/ejcts/ezz267.
Ferraris VA, Ferraris SP, Saha SP, et al. Perioperative blood transfusion and blood conservation in cardiac
surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007; 83: S27-S86. doi: 10.1016/j.athoracsur.2007.02.099.
Gaynor JW. Use of ultrafiltration during and after cardiopulmonary bypass in children. J Thorac Cardiovasc Surg. 2001; 122(2): 209-11. doi: 10.1067/mtc.2001.115925.
Minton J, Sidebotham DA, Hyperlactatemia and cardiac surgery. J Extra Corpor Technol. 2017; 49(1): 7-15.
Ranucci M, De Toffol B, Isgrò G, et al. Hyperlactatemia during cardiopulmonary bypass: determinants and impact on postoperative outcome. Crit Care. 2006; 10(6): 1-9. doi: 10.1186/cc5113.
Alam SR, Stirrat C, Spath N, et al. Myocardial inflammation, injury and infarction during on-pump coronary artery bypass graft surgery. J Cardiothorac Surg. 2017; 12: 1-10. doi.org/10.1186/s13019-017- 0681-6.
Levraut J, Ciebiera JP, Jambou P, et al. Effect of continuous venovenous hemofiltration with dialysis on lactate clearance in critically ill patients. Crit Care Med. 1997; 25(1): 58-62. doi: 10.1097/00003246-199701000-00013.
Rabie Soliman EF, Belghith M, Abdelmageed T. Conventional hemofiltration during cardiopulmonary bypass increases the serum lactate level in adult cardiac surgery. Ann Card Anaesth. 2016; 19(1): 45- 51. doi: 10.4103/0971-9784.173019.
Li Y, Li Y, Hu Q, et al. Association of early elevated cardiac troponin I concentration and longitudinal change after off-pump coronary artery bypass grafting and adverse events: a prospective cohort study. J Thorac Dis. 2020; 12(11): 6542-51. doi: 10.21037/jtd-20-1691.
Tevaearai Stahel HT, Do PD, Klaus JB, et al. Clinical relevance of troponin T profile following cardiac surgery. Front Cardiovasc Med. 2018; 5: 182. doi.org/10.3389/fcvm.2018.00182.