Decelerate amyloid fibrillation by the alkaloids extracted from Stephania venosa
Main Article Content
Abstract
Background: Naturally occurring phytochemical compounds have received considerable attention as alternative candidates for anti-amyloidogenic agents.
Objectives: This study, utilizing human insulin and amyloid-β peptide as an in vitro model, determined the anti-amyloid effects of alkaloids extracted derived from Stephania venosa.
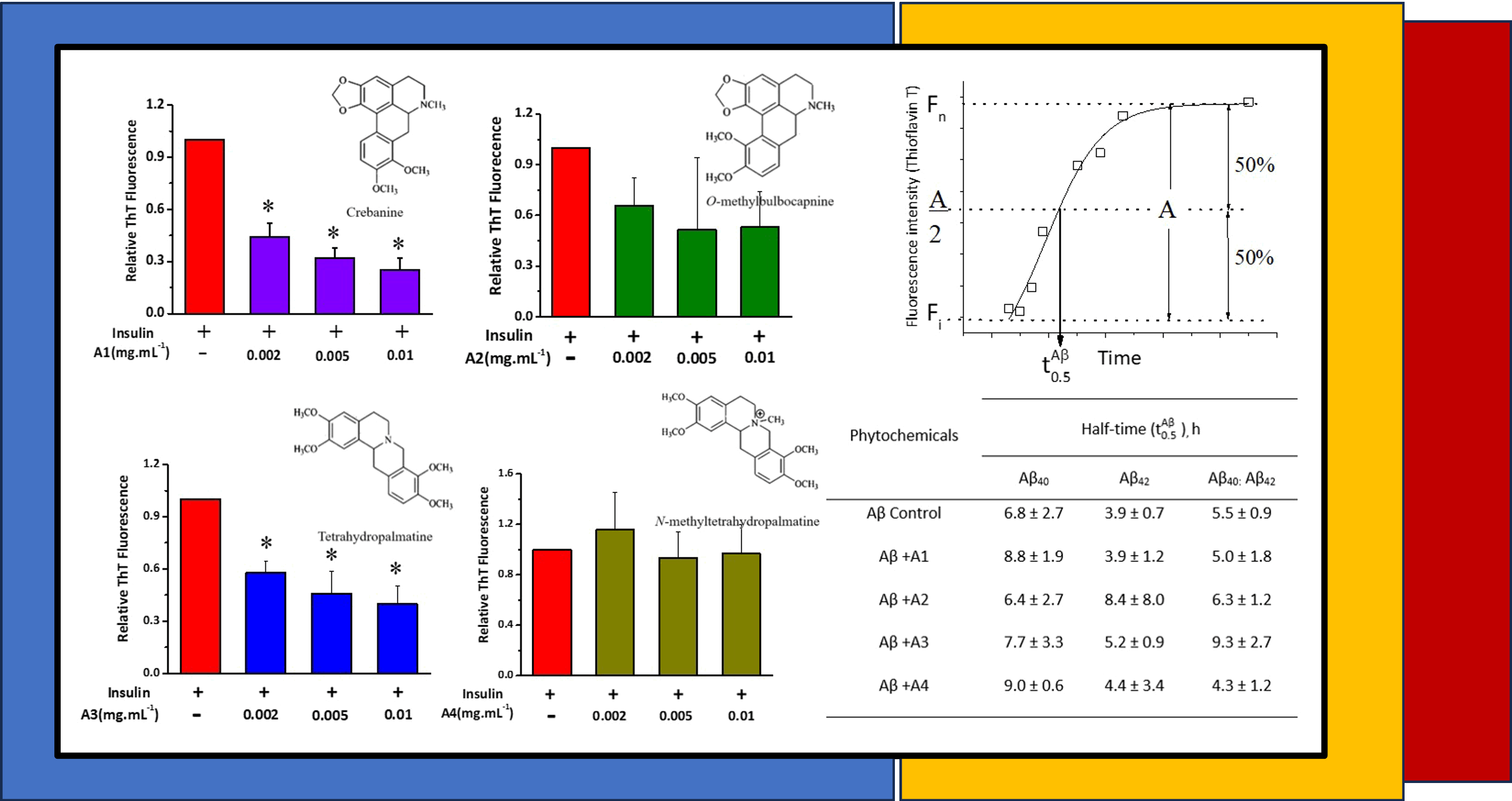
Materials and methods: Alkaloids extracts including crebanine, O-methylbulbocapnine, tetrahydropalmatine and N-methyltetrahydropalmatine were used. The Inhibition of amyloid protein aggregation was studied by fluorescence spectroscopy.
Results: Most alkaloids, except N-methyltetrahydropalmatine, exhibited inhibitory properties against amyloid fibrillation either insulin or amyloid-beta peptide. Among the alkaloids group, crebanine and tetrahydropalmatine showed potent properties of anti-amyloidogenesis.
Conclusion: These results suggest that alkaloids could be used as a natural compound for the development of drugs against amyloid protein aggregation for the treatment of amyloid-related diseases.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Velander P, Wu L, Henderson F, Zhang S, Bevan DR, Xu B. Natural product-based amyloid inhibitors. Biochem Pharmacol. 2017; 139: 40-55. doi: 10.1016/j.bcp.2017.04.004.
Gong H, He Z, Peng A, Zhang X, Cheng B, Sun Y, et al. Effects of several quinones on insulin aggregation. Sci Rep. 2014; 4: 5648. doi: org/10.1038/srep05648.
Wang J-B, Wang Y-M, Zeng C-M. Quercetin inhibits amyloid fibrillation of bovine insulin and destabilizes preformed fibrils. Biochem Biophys Res Commun. 2011; 415(4): 675-9. doi: 10.1016/j.bbrc.2011.10.135.
Ng YP, Or TCT, Ip NY. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochem Int. 2015; 89: 260-70. doi: 10.1016/j.neuint.2015.07.018.
Amirkia V, Heinrich M. Alkaloids as drug leads – A predictive structural and biodiversity-based analysis. Phytochem Lett. 2014; 10: xlviii-liii. doi: 10.1016/j.phytol.2014.06.015.
Matharu B, Gibson G, Parsons R, Huckerby TN, Moore SA, Cooper LJ, et al. Galantamine inhibits β-amyloid aggregation and cytotoxicity. J Neurol Sci. 2009; 280(1): 49-58. doi: 10.1016/j.jns.2009.01.024.
Wilcock G, Howe I, Coles H, Lilienfeld S, Truyen L, Zhu Y, et al. A long-term comparison of galantamine and donepezil in the treatment of Alzheimer’s disease. Drugs Aging. 2003; 20(10): 777-89. doi: 10.2165/00002512-200320100-00006.
Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, et al. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain β-amyloid production. Neuroscience. 2006; 142(4): 941-52. doi:10.1016/j.neuroscience.2006.07.021.
Dall’Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR. Caffeine and adenosine A2a receptor antagonists prevent β-amyloid (25-35)-induced cognitive deficits in mice. Exp Neurol. 2007; 203(1): 241-5. doi: 10.1016/j.expneurol.2006.08.008.
Kongkiatpaiboon S, Duangdee N, Prateeptongkum S, Tayana N, Inthakusol W. Simultaneous HPLC analysis of crebanine, dicentrine, stephanine and tetrahydropalmatine in Stephania venosa. Rev Bras Farmacogn. 2017; 27(6): 691-7. doi: 10.1016/j.bjp.2017.10.004.
Semwal DK, Badoni R, Semwal R, Kothiyal SK, Singh GJP, Rawat U. The genus Stephania (Menispermaceae): chemical and pharmacological perspectives. J Ethno pharmacol. 2010; 132(2): 369-83. doi: 10.1016/j.jep.2010.08.047.
Le PM, Srivastava V, Nguyen TT, Pradines B, Madamet M, Mosnier J, et al. Stephanine from Stephania venosa (Blume) Spreng showed effective antiplasmodial and anticancer activities, the latter by inducing apoptosis through the reverse of mitotic exit. Phytother Res. 2017; 31(9): 1357-68. doi: 10.1002/ptr.5861.
Nantapap S, Loetchutinat C, Meepowpan P, Nuntasaen N, Pompimon WJoPaP. Antiproliferative effects of alkaloids isolated from the tuber of Stephania venosa via the induction of cell cycle arrest in mammalian cancer cell lines. Am J Appl Sci. 2010; 7(8): 1057-65. doi:10.3844/ajassp.2010.1057.1065.
Mon MT, Yodkeeree S, Punfa W, Pompimon W, Limtrakul P. Alkaloids from Stephania venosaas chemosensitizers in SKOV3 ovarian cancer cells via Akt/NF-κB signaling. Chem Pharm Bull. 2018; 66(2): 162-9. doi: 10.1248/cpb.c17-00687.
Ingkaninan K, Phengpa P, Yuenyongsawad S, Khorana N. Acetylcholinesterase inhibitors from Stephania venosa tuber. J Pharm Pharmacol. 2006; 58(5): 695-700. doi: 10.1211/jpp.58.5.0015.
Yodkeeree S, Wongsirisin P, Pompimon W, Limtrakul P. Anti-invasion effect of Crebanine and O-Methylbulbocapnine from Stephania venosavia down-regulated matrix metalloproteinases and urokinase plasminogen activator. Chem Pharm Bull. 2013; 61(11): 1156-65. doi: 10.1248/cpb.c13-00584.
Wongsirisin P, Yodkeeree S, Pompimon W, Limtrakul P. Induction of G1 arrest and apoptosis in human cancer cells by Crebanine, an alkaloid from Stephania venosa. Chem Pharm Bull. 2012; 60(10): 1283-9. doi: 10.1248/cpb.c12-00506.
Yodkeeree S, Pompimon W, Limtrakul P. Crebanine, an aporphine alkaloid, sensitizes TNF-α-induced apoptosis and suppressed invasion of human lung adenocarcinoma cells A549 by blocking NF-κBregulated gene products. Tumor Biol. 2014; 35(9): 8615-24. doi: 10.1007/s13277-014-1998-6.
Oh YC, Choi JG, Lee YS, Brice OO, Lee SC, Kwak HS, et al. Tetrahydropalmatine inhibits pro-inflammatory mediators in lipopolysaccharide-stimulated THP-1 cells. J Med Food. 2010; 13(5): 1125-32. doi: 10.1089/jmf.2009.1388.
Bekard IB, Dunstan DE. Tyrosine autofluorescence as a measure of bovine insulin fibrillation. Biophys J. 2009; 97(9): 2521-31. doi: 10.1016/j.bpj.2009.07.064.
Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, et al. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996; 17(5): 1005-13. doi: 10.1016/s0896-6273(00)802305.
Stewart KL, Radford SE. Amyloid plaques beyond Aβ: a survey of the diverse modulators of amyloid aggregation. Biophys Rev. 2017; 9(4): 405-19. doi: 10.1007/s12551-017-0271-9.
Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014; 4: 177. doi: 10.3389/fphar.2013.00177.
Habtemariam S. Natural products in Alzheimer’s disease therapy: Would old therapeutic approaches fix the broken promise of modern medicines? Molecules. 2019; 24(8): 1519. doi: 10.3390/molecules24081519.
Baptista FI, Henriques AG, Silva AMS, Wiltfang J, da Cruz e Silva OAB. Flavonoids as therapeutic compounds targeting key proteins involved in Alzheimer’s disease. ACS Chem Neurosci. 2014; 5(2): 83-92. doi: 10.1021/cn400213r.
Iannuzzi C, Borriello M, Portaccio M, Irace G, Sirangelo I. Insights into insulin fibril assembly at physiological and acidic pH and related amyloid intrinsic fluorescence. Int J Mol Sci. 2017; 18(12): 2551. doi: 10.3390/ijms18122551.
Lee C-C, Nayak A, Sethuraman A, Belfort G, McRae GJ. A three-stage kinetic model of amyloid fibrillation. Biophys J. 2007; 92(10): 3448-58. doi: 10.1529/biophysj.106.098608.
Muzaffar M, Ahmad A. The mechanism of enhanced insulin amyloid fibril formation by NaCl is better explained by a conformational change model. PloS one. 2011; 6(11): e27906-e. doi: 10.1371/journal.pone.0027906.
Gu L, Guo Z. Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J Neurochem. 2013; 126(3): 305-11. doi: 10.1111/jnc.12202.
Pauwels K, Williams TL, Morris KL, Jonckheere W, Vandersteen A, Kelly G, et al. Structural basis for increased toxicity of pathological aβ42:aβ40 ratios in Alzheimer disease. J Biol Chem. 2012; 287(8): 5650-60. doi: 10.1074/jbc.M111.264473.