Determination of the JAK2 V617F mutation in thrombosis patients
Main Article Content
Abstract
Background: Janus kinase 2 (JAK2) gene mutation causes uncontrolled myeloproliferation independent of cytokines and abnormal formation of the endogenous erythroid colony. JAK2 mutations were frequently observed in myeloproliferative neoplasms (MPNs), especially in polycythemia vera (PV) and essential thrombocythemia (ET). MPNs represent a risk factor for the development of thrombosis that is a significant cause of morbidity and mortality in patients.
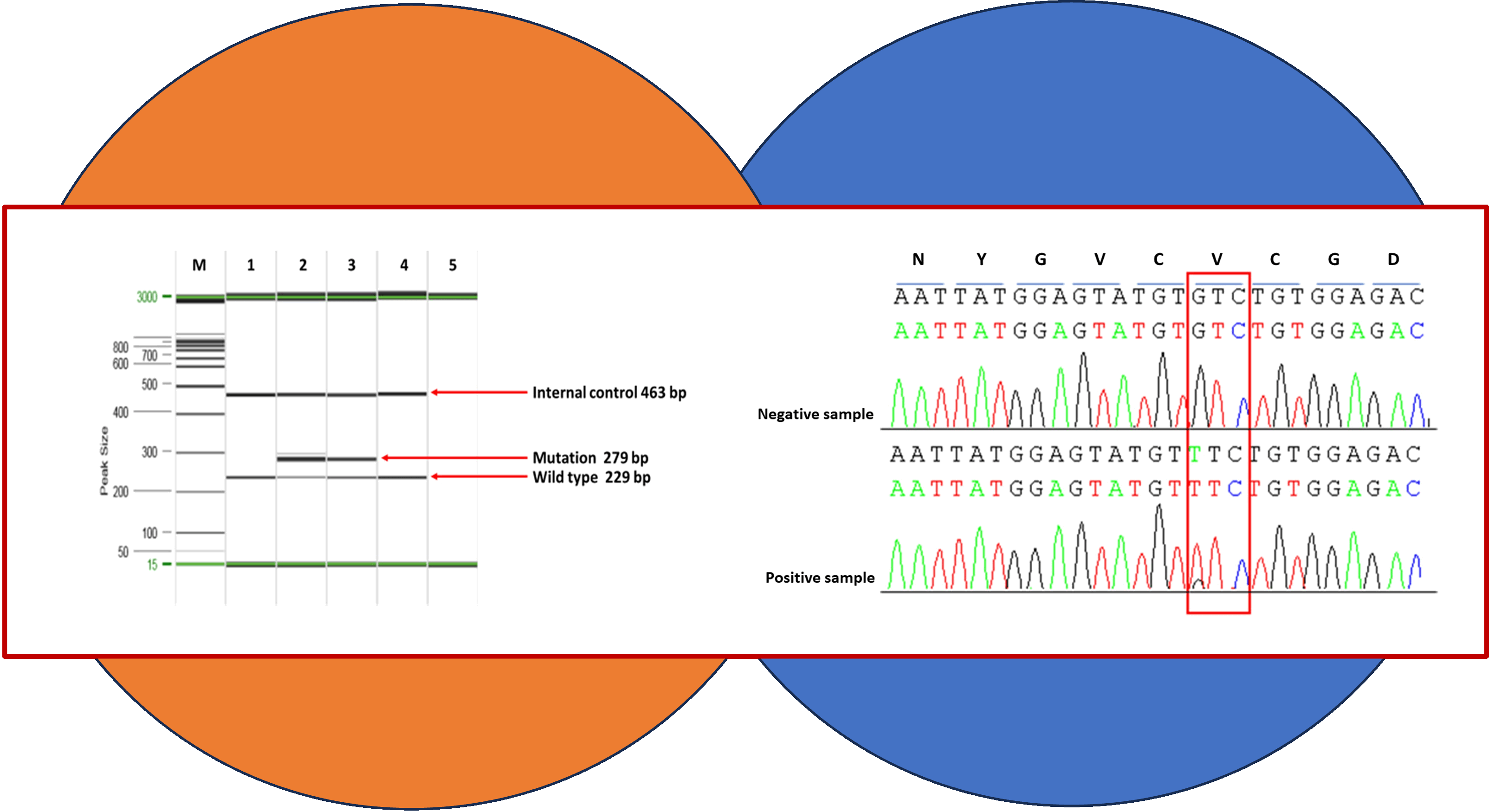
Materials and methods: We aimed to study the correlation between JAK2 mutation and thrombosis. Thirty-nine patients with a clinical diagnosis of thrombocytosis and Budd-Chiari syndrome were collected to determine the JAK2 V617F mutation. Genomic DNA from all specimens was amplified and detected the presence of JAK2 V617F mutation by AS-PCR.
Results: We demonstrated JAK2 V617F mutation in both patients with a clinical diagnosis of thrombocytosis and Budd-Chiari syndrome. We found that 11 of 37 (29.7%) thrombocytosis patients had a JAK2 V617F mutation. Moreover, one of two patients who represented as Budd-Chiari syndrome was positive for JAK2 V617F mutation. In addition, JAK2 V617F mutation was associated with thrombosis. However, further study in large series is needed to support this finding.
Conclusion: Determination of the JAK2 V617F mutation may be helpful for screening latent or occult MPNs patients who have an occurrence of thrombosis to adjust the appropriate treatment for good patient outcomes.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005; 365(9464): 1054-61. doi: 10.1016/S0140-6736(05)71142-9.
Kralovics R, Passamonti F, Buser AS, et al. A gain-offunction mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005; 352(17): 1779-90. doi: 10.1056/NEJMoa051113.
Singdong R, Siriboonpiputtana T, Chareonsirisuthigul T, et al. Characterization and Prognosis Significance of JAK2 (V617F), MPL, and CALR Mutations in Philadelphia-Negative Myeloproliferative Neoplasms. Asian Pac J Cancer Prev. 2016; 17(10): 4647-53. doi: 10.22034/APJCP.2016.17.10.4647.
Larsen TS, Pallisgaard N, Moller MB, Hasselbalch HC. The JAK2 V617F allele burden in essential thrombocythemia, polycythemia vera and primary myelofibrosis--impact on disease phenotype. Eur J Haematol. 2007; 79(6): 508-15. doi: 10.1111/j.16000609.2007.00960.x.
Ha JS, Kim YK, Jung SI, Jung HR, Chung IS. Correlations between Janus kinase 2 V617F allele burdens and clinicohematologic parameters in myeloproliferative neoplasms. Ann Lab Med. 2012; 32(6): 385-91. doi: 10.3343/alm.2012.32.6.385.
Hunger SP, Mullighan CG. Redefining ALL classification: toward detecting high-risk ALL and implementing precision medicine. Blood. 2015; 125(26): 3977-87. doi: 10.1182/blood-2015-02-580043.
Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014; 371(11): 1005-15. doi: 10.1056/NEJMoa1403088.
Roberts KG, Gu Z, Payne-Turner D, et al. High Frequency and Poor Outcome of Philadelphia Chromosome-Like Acute Lymphoblastic Leukemia in Adults. J Clin Oncol. 2017; 35(4): 394-401. doi: 10.1200/JCO.2016.69.0073.
Perner F, Perner C, Ernst T, Heidel FH. Roles of JAK2 in Aging, Inflammation, Hematopoiesis and Malignant Transformation. Cells. 2019; 8(8). doi: 10.3390/cells8080854.
Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004; 350(2): 114-24. doi: 10.1056/NEJMoa035572.
Yesilova AM, Yavuzer S, Yavuzer H, et al. Analysis of thrombosis and bleeding complications in patients with polycythemia vera: a Turkish retrospective study. Int J Hematol. 2017; 105(1): 70-8. doi: 10.1007/s12185-016-2105-0.
Griesshammer M, Kiladjian JJ, Besses C. Thromboembolic events in polycythemia vera. Ann Hematol. 2019; 98(5): 1071-82. doi: 10.1007/s00277-019-03625-x.
Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007; 110(3): 840-6. doi: 10.1182/blood-2006-12-064287.
De Stefano V, Za T, Rossi E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica. 2008; 93(3): 372-80. doi: 10.3324/haematol.12053.
Chung RT, Iafrate AJ, Amrein PC, Sahani DV, Misdraji J. Case records of the Massachusetts General Hospital. Case 15-2006. A 46-year-old woman with sudden onset of abdominal distention. N Engl J Med. 2006; 354(20): 2166-75. doi: 10.1056/NEJMcpc069006.
Smalberg JH, Arends LR, Valla DC, Kiladjian JJ, Janssen HL, Leebeek FW. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood. 2012; 120(25): 4921-8. doi: 10.1182/blood-2011-09-376517.
Yonal I, Pinarbasi B, Hindilerden F, et al. The clinical significance of JAK2V617F mutation for Philadelphianegative chronic myeloproliferative neoplasms in patients with splanchnic vein thrombosis. J Thromb Thrombolysis. 2012; 34(3): 388-96. doi: 10.1007/s11239-012-0738-2.
Colaizzo D, Amitrano L, Guardascione MA, et al. Outcome of patients with splanchnic venous thrombosis presenting without overt MPN: a role for the JAK2 V617F mutation re-evaluation. Thromb Res. 2013; 132(2): e99-e104. doi: 10.1016/j.thromres. 2013.07.014.
Greenfield G, McMullin MF. Splanchnic venous thrombosis in JAK2 V617F mutation positive myeloproliferative neoplasms - long term follow-up of a regional case series. Thromb J. 2018; 16: 33. doi: 10.1186/s12959-018-0187-z.
Colaizzo D, Amitrano L, Tiscia GL, et al. Occurrence of the JAK2 V617F mutation in the Budd-Chiari syndrome. Blood Coagul Fibrinolysis. 2008; 19(5): 459-62. doi: 10.1097/MBC.0b013e3283049662.
Karakose S, Oruc N, Zengin M, Akarca US, Ersoz G. Diagnostic value of the JAK2 V617F mutation for latent chronic myeloproliferative disorders in patients with Budd-Chiari syndrome and/or portal vein thrombosis. Turk J Gastroenterol. 2015; 26(1): 42-48. doi: 10.5152/tjg.2015.5738.
Ma Q. Frequency and characteristics of the JAK2 V617F mutation in 23 cerebral venous sinus thrombosis patients with thrombocytosis. J Int Med Res. 2020; 48(12): 300060520977729. doi: 10.1177/0300060520977729.
Cetin G, Ozkan T, Turgut S, et al. Evaluation of clinical and laboratory findings with JAK2 V617F mutation as an independent variable in essential thrombocytosis. Mol Biol Rep. 2014; 41(10): 6737-42. doi: 10.1007/s11033-014-3559-x.
Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2019 update on diagnosis, risk-stratification and management. Am J Hematol. 2019; 94(1): 133-43. doi: 10.1002/ajh.25303.
Valla D, Casadevall N, Lacombe C, et al. Primary myeloproliferative disorder and hepatic vein thrombosis. A prospective study of erythroid colony formation in vitro in 20 patients with Budd-Chiari syndrome. Ann Intern Med. 1985; 103(3): 329-34. doi: 10.7326/0003-4819-103-3-329.
Valla DC. Budd-Chiari syndrome/hepatic venous outflow tract obstruction. Hepatol Int. 2018; 12 (Suppl 1): 168-80. doi: 10.1007/s12072-017-9810-5.
Amarapurkar DN, Punamiya SJ, Patel ND. Changing spectrum of Budd-Chiari syndrome in India with special reference to non-surgical treatment. World J Gastroenterol. 2008; 14(2): 278-85. doi: 10.3748/wjg.14.278.
Shetty S, Kulkarni B, Pai N, Mukundan P, Kasatkar P, Ghosh K. JAK2 mutations across a spectrum of venous thrombosis cases. Am J Clin Pathol. 2010; 134(1): 82-5. doi: 10.1309/AJCP7VO4HAIZYATP.
Sakr M, Barakat E, Abdelhakam S, et al. Epidemiological aspects of Budd-Chiari in Egyptian patients: a single-center study. World J Gastroenterol. 2011; 17(42): 4704-10. doi: 10.3748/wjg.v17.i42.4704.
Qi X, Zhang C, Han G, et al. Prevalence of the JAK2V617F mutation in Chinese patients with Budd-Chiari syndrome and portal vein thrombosis: a prospective study. J Gastroenterol Hepatol. 2012; 27(6): 1036-43. doi: 10.1111/j.1440-1746.2011.07040.x.
Seijo S, Plessier A, Hoekstra J, et al. Good long-term outcome of Budd-Chiari syndrome with a step-wise management. Hepatology. 2013; 57(5): 1962-8. doi: 10.1002/hep.26306.
Qi X, Han G, Guo X, et al. Review article: the aetiology of primary Budd-Chiari syndrome - differences between the West and China. Aliment Pharmacol Ther. 2016; 44(11-12): 1152-67. doi: 10.1111/apt.13815.
Qi X, Yang Z, Bai M, Shi X, Han G, Fan D. Meta-analysis: the significance of screening for JAK2V617F mutation in Budd-Chiari syndrome and portal venous system thrombosis. Aliment Pharmacol Ther. 2011; 33(10): 1087-103. doi: 10.1111/j.1365-2036.2011.04627.x.