Effect of Thai medicinal plants Acanthus ebracteatus Vahl. Carthamus tinctorius L. and Streblus asper Lour. on neurite outgrowth activity in Neuro-2A cells
Main Article Content
Abstract
Background: Neurite outgrowth is an important process in neural reorganization and repair after neuronal injury. Neurite outgrowth is one of the important mechanisms to maintain normal physiological neuronal function. Neurite stimulation may help to prevent or rehabilitate brain regions in neurodegenerative disease.
Objectives: The aim of this study was to screen selected ethnopharmacological herbs for stimulatory effects on neurite outgrowth and to test for any cytotoxicity and phytochemical properties.
Materials and methods: The herbal extracts derived from Acanthus ebracteatus Vahl. leaves, Carthamus tinctorius L. flower, and Streblus asper Lour. bark was tested for neurite outgrowth stimulation/potentiation and cytotoxic and phytochemical properties.
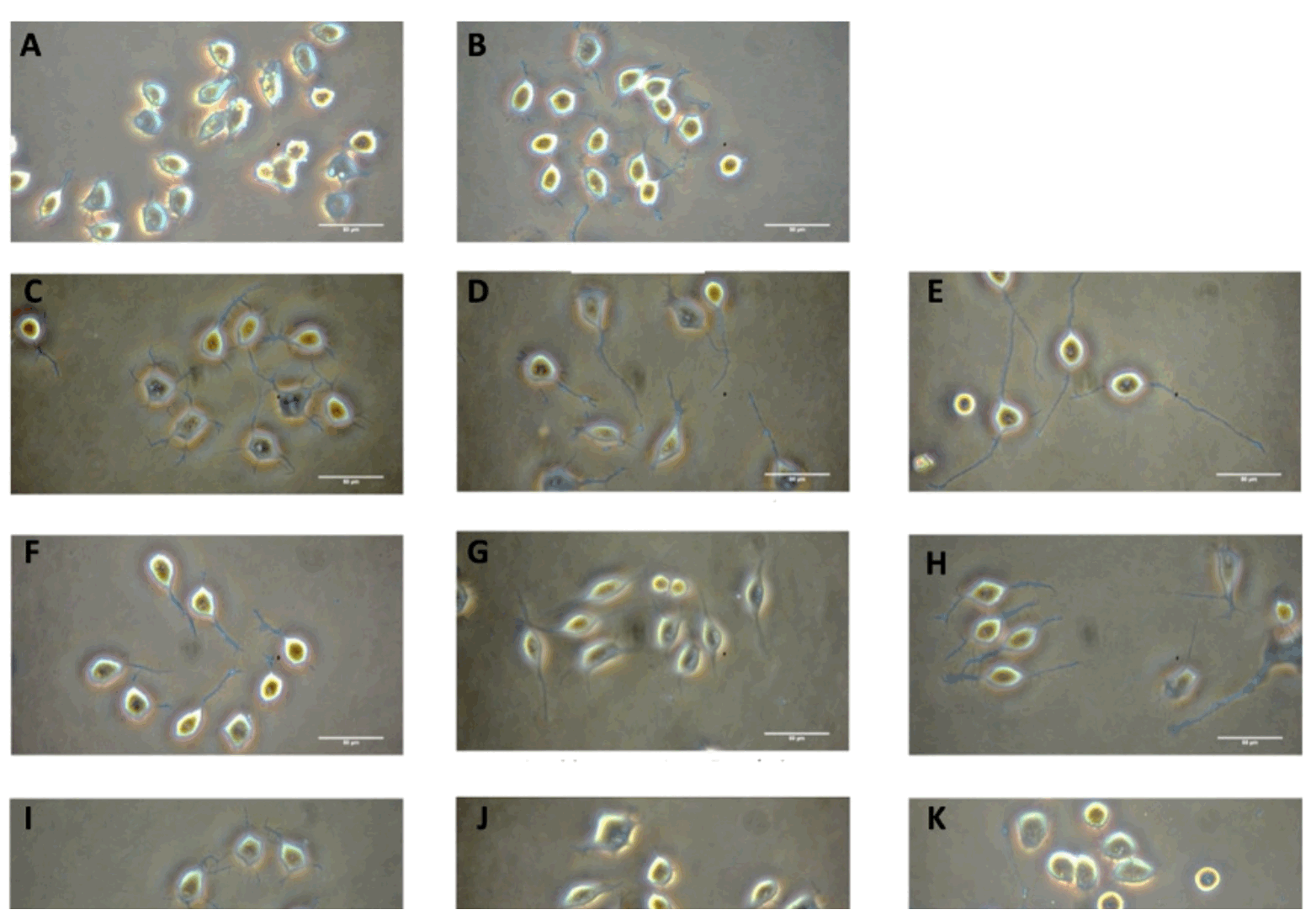
Results: The extract of Carthamus tinctorius L. flowers at concentrations of 50 and 500 µg/mL could significantly stimulate potentiation of neurite outgrowth in Neuro-2a cells whereas other extracts could not. We found that treatment of the cells with a concentration up to 500 µg/mL of the Carthamus tinctorius L. extract showed no cytotoxicity.
Conclusion: The neurite potentiation effect might be due to other chemical constituents rather than phytochemical properties, especially total flavonoid, and phenolic contents, and antioxidant activity of the Carthamus tinctorius L. extract. The result showed that Carthamus tinctorius L. flowers extract could be a good candidate for use as a drug protecting against neuronal damage and neurodegenerative disease since it provides low cytotoxicity and neurogenic enhancement.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nature Reviews Drug Discovery. 2012; 11(3): 191-200.
Waring MJ, Arrowsmith J, Leach AR, Leeson PD, Mandrell S, Owen RM, et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nature reviews Drug Discovery. 2015; 14(7): 475-86.
Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2018; 20(2): 273-86.
Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nature reviews Drug Dscovery. 2004; 3(8): 673-83.
More SV, Koppula S, Kim I-S, Kumar H, Kim B-W, Choi D-K. The Role of Bioactive Compounds on the Promotion of Neurite Outgrowth. Molecules. 2012; 17(6): 6728-53.
Horrigan B, Le Tourneau M. Traditional medicine fact sheet updated by WHO. Alternative Therapies in Health and Medicine. 2003; 9(5): 21.
Brimson JM, Brimson S, Prasanth MI, Thitilertdecha P, Malar DS, Tencomnao T. The effectiveness of Bacopa monnieri (Linn.) Wettst. as a nootropic, neuroprotective, or antidepressant supplement: analysis of the available clinical data. Scientific reports. 2021; 11(1) : 596.
Brimson JM, Prasanth MI, Plaingam W, Tencomnao T. Bacopa monnieri (L.) wettst. Extract protects against glutamate toxicity and increases the longevity of Caenorhabditis elegans. Journal of Traditional and Complementary Medicine. 2020; 10(5): 460-70.
Prince M, Guerchet M, Prina M. The global impact of dementia 2013-2050: Alzheimer's disease international. 2013; London. 1-8.
Dhawan B, Singh H, editors. Pharmacology of Ayurvedic nootropic Bacopa monniera. Proceedings of the International Convention of Biological Psychiatry; 1996.
Brimson JM, Prasanth MI, Isidoro C, Sukprasansap M, Tencomnao T. Cleistocalyx nervosum var. paniala seed extracts exhibit sigma-1 antagonist sensitive neuroprotective effects in PC12 cells and protect C. elegans from stress via the SKN-1/NRF-2 pathway. Nutrition and Healthy Aging. 2021; 6(2): 131-46.
Rangsinth P, Duangjan C, Sillapachaiyaporn C, Isidoro C, Prasansuklab A, Tencomnao T. Caesalpinia mimosoides Leaf Extract Promotes Neurite Outgrowth and Inhibits BACE1 Activity in Mutant APP-Overexpressing Neuronal Neuro2a Cells. Pharmaceuticals. 2021; 14(9): 901.
Prasanth MI, Brimson JM, Sheeja Malar D, Prasansuklab A, Tencomnao T. Streblus asper Lour. exerts MAPK and SKN-1 mediated anti-aging, anti-photoaging activities and imparts neuroprotection by ameliorating Aβ in Caenorhabditis elegans. Nutrition and Healthy Aging. 2021; 6(3): 211-27.
Prasansuklab A, Tencomnao T. Acanthus ebracteatus leaf extract provides neuronal cell protection against oxidative stress injury induced by glutamate. BMC complementary and alternative medicine. 2018; 18(1): 1-15.
Prasansuklab A, Brimson JM, Tencomnao T. Potential Thai medicinal plants for neurodegenerative diseases: A review focusing on the anti-glutamate toxicity effect. Journal of Traditional and Complementary Medicine. 2020; 10(3): 301-8.
Yang Q, Yang Z-F, Liu S-B, Zhang X-N, Hou Y, Li X-Q, et al. Neuroprotective effects of hydroxysafflor yellow A against excitotoxic neuronal death partially through down-regulation of NR2B-containing NMDA receptors. Neurochemical research. 2010; 35(9): 1353-60.
Chu D, Liu W, Huang Z, Liu S, Fu X, Liu K. Pharmacokinetics and excretion of hydroxysafflor yellow A, a potent neuroprotective agent from safflower, in rats and dogs. Planta medica. 2006; 72(05): 418-23.
Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry. 1999; 64(4): 555-9.
Brimson JM, Brimson SJ, Brimson CA, Rakkhitawatthana V, Tencomnao T. Rhinacanthus nasutus extracts prevent glutamate and amyloid-β neurotoxicity in HT-22 mouse hippocampal cells: possible active compounds include lupeol, stigmasterol and β-sitosterol. International journal of molecular sciences. 2012; 13(4): 5074-97.
Singleton VL, Rossi JA. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. American Journal of Enology and Viticulture. 1965; 16(3): 144-58.
Duangjan C, Rangsinth P, Gu X, Wink M, Tencomnao T. Lifespan Extending and Oxidative Stress Resistance Properties of a Leaf Extracts from Anacardium occidentale L. in Caenorhabditis elegans. Oxidative Medicine and Cellular Longevity. 2019; 2019: 9012396.
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999; 26(9-10): 1231-7.
Pattarachotanant N, Tencomnao T. Citrus hystrix Extracts Protect Human Neuronal Cells against High Glucose-Induced Senescence. Pharmaceuticals. 2020; 13(10): 283.
Brimson JM, Safrany ST, Qassam H, Tencomnao T. Dipentylammonium Binds to the Sigma-1 Receptor and Protects Against Glutamate Toxicity, Attenuates Dopamine Toxicity and Potentiates Neurite Outgrowth in Various Cultured Cell Lines. Neurotoxicity Research. 2018; 34(2): 263-72.
Jin Y, Xiao Y-s, Zhang F-f, Xue X-y, Xu Q, Liang X-m. Systematic screening and characterization of flavonoid glycosides in Carthamus tinctorius L. by liquid chromatography/UV diode-array detection/electrospray ionization tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2008; 46(3): 418-30.
Zhou X, Tang L, Xu Y, Zhou G, Wang Z. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: A phytochemical and pharmacological review. Journal of Ethnopharmacology. 2014; 151(1) :27-43.
Afendi FM, Okada T, Yamazaki M, Hirai-Morita A, Nakamura Y, Nakamura K, et al. KNApSAcK family databases: integrated metabolite-plant species databases for multifaceted plant research. Plant Cell Physiol. 2012; 53(2): e1.
Whittle M, Willett P, Klaffke W, van Noort P. Evaluation of similarity measures for searching the dictionary of natural products database. J Chem Inf Comput Sci. 2003;43(2):449-57.
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009; 30(16): 2785-91.
Liu C, Chan CB, Ye K. 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Translational Neurodegeneration. 2016;5.
Chitranshi N, Gupta V, Kumar S, Graham SL. Exploring the Molecular Interactions of 7,8-Dihydroxyflavone and Its Derivatives with TrkB and VEGFR2 Proteins. International Journal of Molecular Sciences. 2015; 16(9): 21087-108.
Michel T, Halabalaki M, Skaltsounis AL. New concepts, experimental approaches, and dereplication strategies for the discovery of novel phytoestrogens from natural sources. Planta Med. 2013; 79(7): 514-32.
Pang J, Hou J, Zhou Z, Ren M, Mo Y, Yang G, et al. Safflower yellow improves synaptic plasticity in APP/PS1 mice by regulating microglia activation phenotypes and BDNF/TrkB/ERK signaling pathway. NeuroMolecular Medicine. 2020; 22(3): 341-58.
Gupta VK, You Y, Gupta VB, Klistorner A, Graham SL. TrkB receptor signalling: implications in neurodegenerative, psychiatric and proliferative disorders. International Journal of Molecular Sciences. 2013; 14(5): 10122-42.
Fumagalli F, Racagni G, Riva MA. Shedding light into the role of BDNF in the pharmacotherapy of Parkinson's disease. The Pharmacogenomics Journal. 2006; 6(2): 95-104.
Miranda M, Morici JF, Zanoni MB, Bekinschtein P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front Cell Neurosci. 2019; 13: 363.
Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Grãos MM, et al. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death & Differentiation. 2005; 12(10): 1329-43.
Thoenen H, Sendtner M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat Neurosci. 2002; 5(11): 1046-50.
Ochs G, Penn RD, York M, Giess R, Beck M, Tonn J, et al. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000; 1(3): 201-6.
Wang L, Botchway BO, Liu X. The Repression of the HMGB1-TLR4-NF-κB Signaling Pathway by Safflower Yellow May Improve Spinal Cord Injury. Frontiers in Neuroscience. 2021; 15.
Wang T, Wang L, Li C, Han B, Wang Z, Li J, et al. Hydroxysafflor yellow A improves motor dysfunction in the rotenone-induced mice model of Parkinson’s disease. Neurochemical research. 2017; 42(5): 1325-32.
Delshad E, Yousefi M, Sasannezhad P, Rakhshandeh H, Ayati Z. Medical uses of Carthamus tinctorius L. (Safflower): a comprehensive review from Traditional Medicine to Modern Medicine. Electron Physician. 2018; 10(4): 6672-81.
Salem N, Msaada K, Elkahoui S, Mangano G, Azaeiz S, Ben Slimen I, et al. Evaluation of antibacterial, antifungal, and antioxidant activities of safflower natural dyes during flowering. Biomed Res Int. 2014; 2014: 762397.
Duan JL, Wang JW, Guan Y, Yin Y, Wei G, Cui J, et al. Safflor yellow A protects neonatal rat cardiomyocytes against anoxia/reoxygenation injury in vitro. Acta Pharmacologica Sinica. 2013; 34(4): 487-95.
Innis SM, de la Presa Owens S. Dietary Fatty Acid Composition in Pregnancy Alters Neurite Membrane Fatty Acids and Dopamine in Newborn Rat Brain. The Journal of Nutrition. 2001; 131(1): 118-22.