Influence of low-dose X-ray on plasma membrane properties of erythroleukemia cell lines (K562, K562/adr)
Main Article Content
Abstract
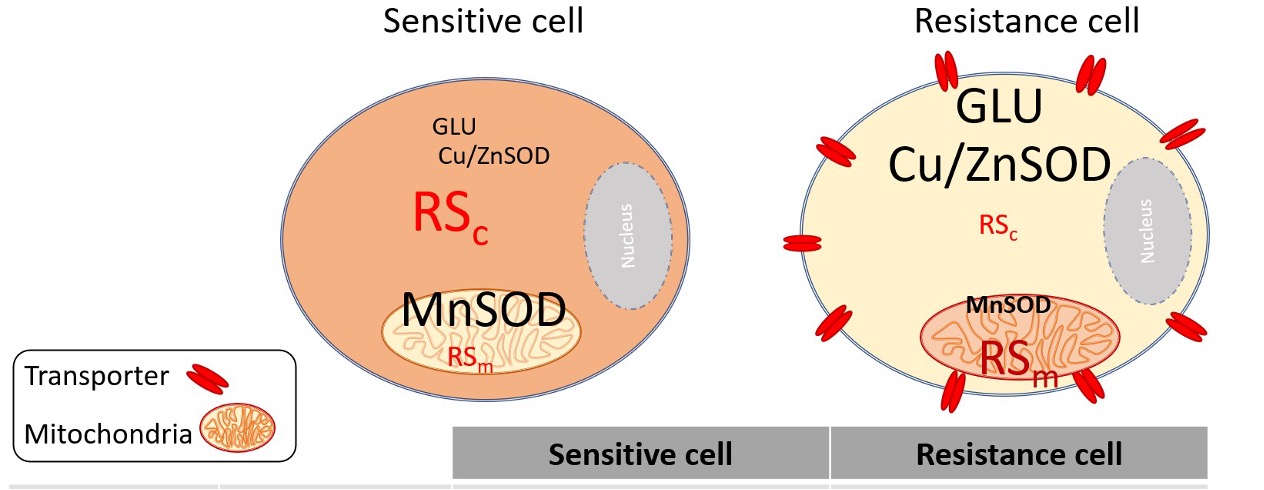
Background: Low-dose X-ray in medical use for diagnosis and therapy can result in cellular biology either directly or indirectly. In cell biology, the interaction of low-dose radiation generates many radical molecules that interact with cellular organelles, such as the plasma membrane.
Objectives: This study aimed to evaluate the effect of low-dose X-ray on both drug-sensitive (K562) and drug-resistant (K562/adr) erythroleukemic cell lines.
Materials and methods: Cells were exposed by using an X-ray at 135 kVp to obtain the absorbed dose of 0.05, 0.1, and 0.2 Gy. The intracellular reactive oxidant species (RS), malondialdehyde, membrane fluidity, drug uptake, and drug accumulation were instantly observed after radiation.
Results: The result showed a significant increase in RS in both cell lines as a function of radiation dose. In K562, the malondialdehyde (MDA) value increased in a radiation dose manner, while membrane fluidity was significantly modified at 0.1 and 0.2 Gy. In K562/adr, the uptake rate of pirarubicin (THP) and IC20 were altered but not significantly different from sham control.
Conclusion: Low-dose X-ray significantly increased the intracellular RS in both cell lines and decreased the membrane fluidity at 0.1 Gy of K562. There are alterations of anticancer drug uptake rate in both cell lines, but they are not significant.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Personal views expressed by the contributors in their articles are not necessarily those of the Journal of Associated Medical Sciences, Faculty of Associated Medical Sciences, Chiang Mai University.
References
Mettler FA, Jr., Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008; 248(1): 254-63.
Schröder A, Kriesen S, Hildebrandt G, Manda K. First insights into the effect of low-dose X-ray irradiation in adipose-derived stem cells. Int J Mol Sci. 2019; 20(23): 6075.
Kirkby C, Mackenzie M. Is low dose radiation therapy a potential treatment for COVID-19 pneumonia? Radiother Oncol. 2020; 147: 221.
Nagashima H, Shiraishi K, Ohkawa S, Sakamoto Y, Komatsu K, Matsuura S, et al. Induction of somatic mutations by low-dose X-rays: the challenge in recognizing radiation-induced events. J Radiat Res. 2018; 59(suppl_2): ii11-ii7.
Ayala A, Muñoz M, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid Med Cell Longev. 2014; 2014: 1-31.
Kolling A, Maldonado C, Ojeda F, Diehl HA. Membrane fluidity of microsomal and thymocyte membranes after X-ray and UV irradiation. Radiation and environmental biophysics. 1994; 33(4): 303-13.
Pignatello R, Musumeci T, Basile L, Carbone C, Puglisi G. Biomembrane models and drug-biomembrane interaction studies: Involvement in drug design and development. J Pharm Bioallied Sci. 2011; 3(1): 4-14.
Pochano S, Noitana K, Tungjai M, Udomtanakunchai C. Effects of Low-Dose X-ray on Oxidative State, Lipid Peroxidation, and Membrane Fluidity of Human Peripheral Blood Mononucleated Cells. Journal of Associated Medical Sciences. 2019; 52(3): 193-8.
Tungjai M, Phathakanon N, Ketnuam P, Tinlapat J, Kothan S. Determination of hemolysis, osmotic fragility and fluorescence anisotropy on irradiated red blood cells as a function of kV of medical diagnostic X-rays. International Journal of Radiation Research. 2018; 16(1): 123-7.
Alves AC, Ribeiro D, Nunes C, Reis S. Biophysics in cancer: the relevance of drug-membrane interaction studies. Biochim Biophys Acta. 2016; 1858(9): 2231-44.
Supawat B, Thammathikornchai P, Sutinkat Y, Tima S, Udomtanakunchai C, Kothan S, et al. Influence of short-term iodinated radiographic contrast media exposure on reactive oxygen species levels in K562 cancer cells. J Assoc Med Sci. 2019; 52(2): 96-102.
Loetchutinat C, Kothan S, Dechsupa S, Meesungnoen J, Jay-Gerin JP, Mankhetkorn S. Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant cancer cells using the 2',7'-dichlorofluorescein diacetate assay. Radiat Phys Chem. 2005; 72(2-3): 323-31.
da Silva JK, Cazarin CBB, Batista ÂG, Maróstica M. Effects of passion fruit (Passiflora edulis) byproduct intake in antioxidant status of Wistar rats tissues. LWT - Food Science and Technology. 2014; 59(2, Part 2): 1213-9.
Draper HH, Hadley M. Malondialdehyde determination as index of lipid Peroxidation. Oxygen Radicals in Biological Systems Part B: Oxygen Radicals and Antioxidants. Methods in Enzymology. 186. Cambridge, Massachusetts: Academic Press; 1990. p. 421-31.
Gidwani A, Holowka D, Baird B. Fluorescence anisotropy measurements of lipid order in plasma membranes and lipid rafts from RBL-2H3 mast cells. Biochemistry. 2001; 40(41): 12422-9.
Garnier-Suillerot A, Marbeuf-Gueye C, Salerno M, Loetchutinat C, Fokt I, Krawczyk M, et al. Analysis of drug transport kinetics in multidrug-resistant cells: Implications for drug action. Curr Med Chem. 2001; 8(1): 51-64.
Czekanska EM. Assessment of cell proliferation with resazurin-based fluorescent dye. Methods in molecular biology (Clifton, NJ). 2011; 740: 27-32.
Udomtanakunchai C, Mankhetkorn S. Determination of molecular markers for diagnosis of cancers and for cancer cell response to polyphenol treatment in Xenografted rats by using NMR, MRS and MRI in combination with biochemical analysis. Thailand Research Fund, Thailand; 2012. Report No.: MRG5080217.
Jancam S. Determination of manganese superoxide dismutase in normal and cancer cells by native polyacrylamide gel electrophoresis. Chiang Mai, Thailand: Chiang Mai University; 2011.
Indo HP, Inanami O, Koumura T, Suenaga S, Yen HC, Kakinuma S, et al. Roles of mitochondria-generated reactive oxygen species on X-ray-induced apoptosis in a human hepatocellular carcinoma cell line, HLE. Free Radic Res. 2012; 46(8): 1029-43.
Joksic G, Pajovic Sb Fau - Stankovic M, Stankovic M Fau - Pejic S, Pejic S Fau - Kasapovic J, Kasapovic J Fau - Cuttone G, Cuttone G Fau - Calonghi N, et al. Chromosome aberrations, micronuclei, and activity of superoxide dismutases in human lymphocytes after irradiation in vitro. Cell Mol Life Sci. 2000; (57): 842-50.
Pajović SB, Joksić G Fau - Kasapović J, Kasapović J Fau - Pejić S, Pejić S Fau - Kanazir DT, Kanazir DT. Role of antioxidant enzymes in radiosensitivity of human blood cells. J Environ Pathol Toxicol Oncol. 2000; 19(4): 325-31.
Eken A, Aydin A, Erdem O, Akay C, Sayal A, Somuncu İ. Induced antioxidant activity in hospital staff occupationally exposed to ionizing radiation. Int J Radiat Biol. 2012; 88(9): 648-53.
Asano T, Tsutsuda-Asano A, Fukunaga Y. Indomethacin overcomes doxorubicin resistance by decreasing intracellular content of glutathione and its conjugates with decreasing expression of γ-glutamylcysteine synthetase via promoter activity in doxorubicin-resistant leukemia cells. Cancer Chemother Pharmacol. 2009; 64(4): 715-21.
Navarro J, Obrador E Fau - Pellicer JA, Pellicer Ja Fau - Aseni M, Aseni M Fau - Viña J, Viña J Fau - Estrela JM, Estrela JM. Blood glutathione as an index of radiation-induced oxidative stress in mice and humans. Free Radic Biol Med. 1997; 22(7): 1203-9.
Siegfried JA, Kennedy KA, Sartorelli AC, Tritton TR. The role of membranes in the mechanism of action of the antineoplastic agent adriamycin. Spin-labeling studies with chronically hypoxic and drug-resistant tumor cells. J Biol Chem. 1983; 258(1): 339-43.