An Update on Novel Targeted Treatments for IgA Nephropathy
Main Article Content
Abstract
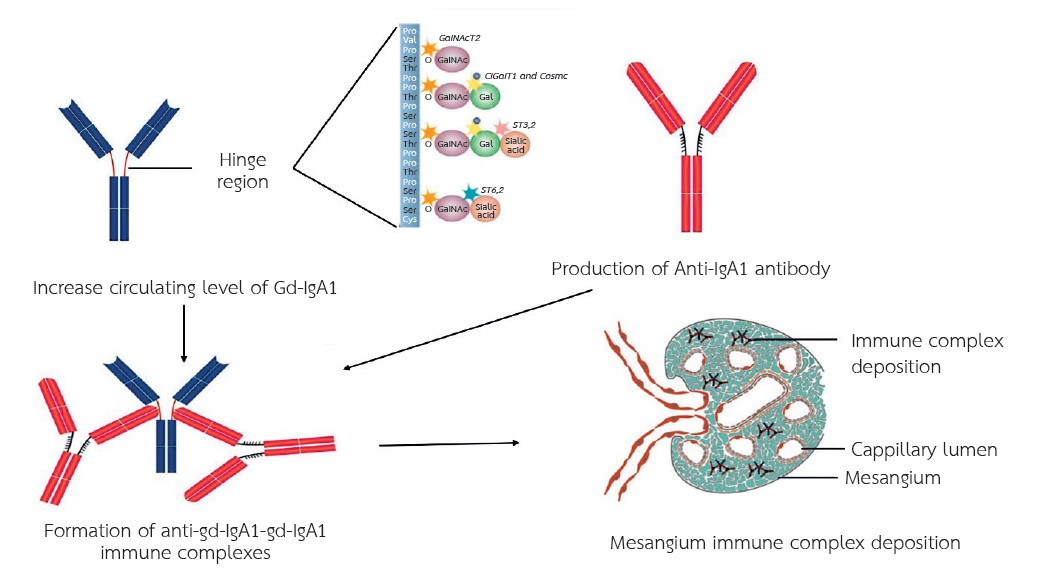
Immunoglobulin A nephropathy (IgAN) stands as the most prevalent primary glomerular disease globally. Its pathogenesis is multifaceted, primarily characterized by elevated circulating levels of Gd-IgA1, which is targeted by autoantibodies. This interaction leads to the formation of circulating immune complexes that subsequently deposit in the glomeruli, triggering intrarenal inflammation. Current therapeutic approaches primarily focus on conservative measures, such as optimized control of blood pressure and proteinuria, aimed at enhancing renal function. Additionally, immunosuppressive therapy, including corticosteroids, may be considered for patients exhibiting persistent proteinuria (>1 g/day) after a minimum of 90 days of conservative management. Recent advancements have led to the development of novel drugs targeting the underlying pathogenic mechanisms of IgAN, particularly those involving immune response and mucosal immunity, with the goal of reducing Gd-IgA1 levels and immune complex deposition in the glomeruli. Furthermore, other treatments, including drugs affecting the complement pathway, endothelin-1 receptor inhibitors, and SGLT2 inhibitors, have demonstrated potential in reducing proteinuria and kidney inflammation. These emerging strategies are promising improving outcomes in IgAN.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This article is published under CC BY-NC-ND 4.0 license, which allows for non-commercial reuse of the published paper as long as the published paper is fully attributed. Anyone can share (copy and redistribute) the material in any medium or format without having to ask permission from the author or the Nephrology Society of Thailand.
References
Rodrigues JC, Haas M, Reich HN. IgA Nephropathy. Clin J Am Soc Nephrol 2017;12:677–86.
Lai KN, Tang SCW, Schena FP, Novak J, Tomino Y, Fogo AB, et al. IgA nephropathy. Nat Rev Dis Primers 2016;2:16001.
Magistroni R, D’Agati VD, Appel GB, Kiryluk K. New developments in the genetics, pathogenesis, and therapy of IgA nephropathy.
Kidney Int 2015;88:974–89.
Boyd JK, Cheung CK, Molyneux K, Feehally J, Barratt J. An update on the pathogenesis and treatment of IgA nephropathy. Kidney Int 2012;81:833–43.
Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int 2021;100:S1–276.
Coppo R, Peruzzi L, Amore A, Piccoli A, Cochat P, Stone R, et al. IgACE: A Placebo-Controlled, Randomized Trial of Angiotensin-Converting Enzyme Inhibitors in Children and Young People with IgA Nephropathy and Moderate Proteinuria. J Am Soc Nephrol 2007;18:1880–8.
Praga M, Gutiérrez E, González E, Morales E, Hernández E. Treatment of IgA Nephropathy with ACE Inhibitors: A Randomized and Controlled Trial. J Am Soc Nephrol 2003;14:1578–83.
Manno C, Torres DD, Rossini M, Pesce F, Schena FP. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant 2009;12:3694–701.
Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, et al. Intensive Supportive Care plus Immunosuppression in IgA Nephropathy. N Engl J Med 2015;373:2225–36.
Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, et al. Effect of Oral Methylprednisolone on Clinical Outcomes in Patients With IgA Nephropathy. JAMA 2017;318:432–42.
Lv J, Wong MG, Hladunewich MA, Jha V, Hooi LS, Monaghan H, et al. Effect of Oral Methylprednisolone on Decline in Kidney Function or Kidney Failure in Patients With IgA Nephropathy. JAMA 2022;327:1888–98.
Reich HN, Floege J. How I Treat IgA Nephropathy. Clin J Am Soc Nephrol 2022;17:1243–6.
Fellström BC, Barratt J, Cook H, Coppo R, Feehally J, de Fijter JW, et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet 2017;389:2117–27.
Barratt J, Lafayette R, Kristensen J, Stone A, Cattran D, Floege J, et al. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int 2023;103(2):391-402
Kim YG, Alvarez M, Suzuki H, Hirose S, Izui S, Tomino Y, et al. Pathogenic Role of a Proliferation-Inducing Ligand (APRIL) in Murine IgA Nephropathy. PLoS One 2015;10(9):e0137044.
Lafayette RA, Canetta PA, Rovin BH, Appel GB, Novak J, Nath KA, et al. A Randomized, Controlled Trial of Rituximab in IgA Nephropathy with Proteinuria and Renal Dysfunction. J Am Soc Nephrol 2017;28:1306–13.
Mathur M, Barratt J, Suzuki Y, Engler F, Pasetti MF, Yarbrough J, et al. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of VIS649 (Sibeprenlimab), an APRIL-Neutralizing IgG2 Monoclonal Antibody, in Healthy Volunteers. Kidney Int Rep 2022;7:993–1003.
Barratt J, Hour B, Sibley C, Mittan A, Roy S, Stromatt C, et al. FC 040 interim results of phase 1 and 2 trials to investigate the safety, tolerability, pharmacokinetics, pharmacodynamics, and clinical activity of BION-1301 in patients with IgA nephropathy. Nephrol Dial Transplant 2021;36 Suppl 1:gfab117.004.
Barratt J, Tumlin J, Suzuki Y, Kao A, Aydemir A, Pudota K, et al. Randomized Phase II JANUS Study of Atacicept in Patients With IgA Nephropathy and Persistent Proteinuria. Kidney Int Rep 2022;7:1831–41.
Bai L, Li H, Li J, Song J, Zhou Y, Liu B, et al. Immunosuppressive effect of artemisinin and hydroxychloroquine combination therapy on IgA nephropathy via regulating the differentiation of CD4+ T cell subsets in rats. Int Immunopharmacol 2019;70:313–23.
Zhang X, Wu X, Xiong L, Yi Z, He Q, He X, et al. Role of vitamin D3 in regulation of T helper cell 17 and regulatory T-cell balance in rats with immunoglobulin a nephropathy. Iran J Kidney Dis 2014;8:363–70.
Gan L, Li X, Zhu M, Chen C, Luo H, Zhou Q. Acteoside relieves mesangial cell injury by regulating Th22 cell chemotaxis and proliferation in IgA nephropathy. Ren Fail 2018;40:364–70.
McAdoo SP, Bhangal G, Page T, Cook TH, Pusey CD, Tam FWK. Correlation of disease activity in proliferative glomerulonephritis with glomerular spleen tyrosine kinase expression. Kidney Int 2015;88:52–60.
Tam WK F, Tumlin J, Barratt J, Rovin H B, Roberts SD I, Roufosse C, et al. SUN-036 spleen tyrosine kinase (SYK) Inhibition in IgA nephropathy: a global, phase II, randomize placebo-controlled trial of FOSTAMATINIB. Kidney Int Rep 2019;4:S168.
Chang A, Ko K, Clark MR. The Emerging Role of the Inflammasome in Kidney Diseases. Curr Opin Nephrol Hypertens 2014;23:204–10.
Qin JH, Lin JR, Ding WF, Wu WH. Schisandrin B Improves the Renal Function of IgA Nephropathy Rats Through Inhibition of the NF-κB Signalling Pathway. Inflammation 2019;42:884–94.
Liu LJ, Yang YZ, Shi SF, Bao YF, Yang C, Zhu SN, et al. Effects of Hydroxychloroquine on Proteinuria in IgA Nephropathy: A Randomized Controlled Trial. Am J Kidney Dis 2019;74:15–22.
Rizk DV, Maillard N, Julian BA, Knoppova B, Green TJ, Novak J, et al. The Emerging Role of Complement Proteins as a Target for Therapy of IgA Nephropathy. Front Immunol 2019;10:504.
Sato S, Yanagihara T, Ghazizadeh M, Ishizaki M, Adachi A, Sasaki Y, et al. Correlation of Autophagy Type in Podocytes with Histopathological Diagnosis of IgA Nephropathy. Pathobiology 2009;76:221–6.
Liu D, Liu Y, Chen G, He L, Tang C, Wang C, et al. Rapamycin Enhances Repressed Autophagy and Attenuates Aggressive Progression in a Rat Model of IgA Nephropathy. Am J Nephrol 2017;45:293–300.
Barratt J, Rovin B, Diva U, Mercer A, Komers R. Implementing the Kidney Health Initiative Surrogate Efficacy Endpoint in Patients With IgA Nephropathy (the PROTECT Trial). Kidney Int Rep 2019;4:1633–7.
Kim SG, Vo N, Lee SH, Ranganathan D, Inker L, El-Shahawy M, et al. FC052: Atrasentan for the Treatment of IGA Nephropathy: Interim Results from the Affinity Study. Nephrol Dial Transplant 2022;37 Suppl 3:gfac107.004.
Vasquez-Rios G. SGLT2 Inhibitors for the Management of IgA Nephropathy: A New Therapeutic Paradigm for an Old Entity. Kidney News 2022;14:22-22.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med 2020;383:1436–46.
Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, et al.Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med 2023;388:117–27.
Nuffield Department of Population Health Renal Studies Group, SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 2022;400:1788–801.
Barratt J, Rovin B, Zhang H, Kashihara N, Maes B, Rizk D, et al. POS-546 Efficacy and safety of Iptacopan in IgA nephropathy: result of a randomized double-blind placebo-controlled phase 2 study at 6 months. Kidney Int Rep 2022;7:S236.
Nesbitt H. American Society of Nephrology | Kidney Week-Abstract Details[Internet].2022 [cited 2023 Jan 16]. Available from: https://www.asn online.org/education/kidneyweek/2022/program-abstract.aspx?controlId=3766973
Lafayette RA, Rovin BH, Reich HN, Tumlin JA, Floege J, Barratt J. Safety, Tolerability and Efficacy of Narsoplimab, a Novel MASP-2 Inhibitor for the Treatment of IgA Nephropathy. Kidney Int Rep 2020;5:2032–41.
Barratt J, Yeo SC, Fernström A, Barbour SJ, Sperati CJ, Villanueva R, et al. Results from the Phase 2 Study of Cemdisiran in Adult Patients with IgA Nephropathy. J Am Soc Nephrol-Abstract Details[Internet].2022 [cite 2023 Jan 16]. Available from: https://www.asn-online.org/education/kidneyweek/2022/program-abstract.aspx?controlId=3797891
Rosenblad T, Rebetz J, Johansson M, Békássy Z, Sartz L, Karpman D. Eculizumab treatment for rescue of renal function in IgA nephropathy. Pediatr Nephrol 2014;29:2225–8.
Bruchfeld A, Magin H, Nachman P, Parikh S, Lafayette R, Potarca A, et al. C5a receptor inhibitor avacopan in immunoglobulin A nephropathy-an open-label pilot study. Clin Kidney J 2022;15:922–8.