The effect of peracetic acid on the corrosion and clearance efficiency of reused dialyzer
Main Article Content
Abstract
Background: A peracetic acid sterilant of dialyzer reuse is a practical approach in hemodialysis of renal replacement therapy. Currently, using of the commercial polyethersulfone (PES), a synthetic polymer-based dialyzer membrane, is increasingly utilized. However, a certain numbers of dialyzer reprocessing has not yet been determined as a standard guideline even the capacity of sterilized dialyzer can be affected by the corrosion of peracetic acid.
Method: The physical and chemical characteristics of the PES membranes within dialyzers were evaluated using scanning electron microscope (SEM) and energy dispersive X-ray spectroscopy (EDS) in three female patients with end stage renal disease aged 50-65 years. The parameters of hemodialysis efficiency were also examined. Fifteen dialyzers were allocated into the following two groups as 1) three of new dialyzers (the reuse number is zero) and 2) reuse of dialyzers undergo the number of reuses increases as 1, 5, 10, and 15 times (three dialyzers for each sub-group), respectively.
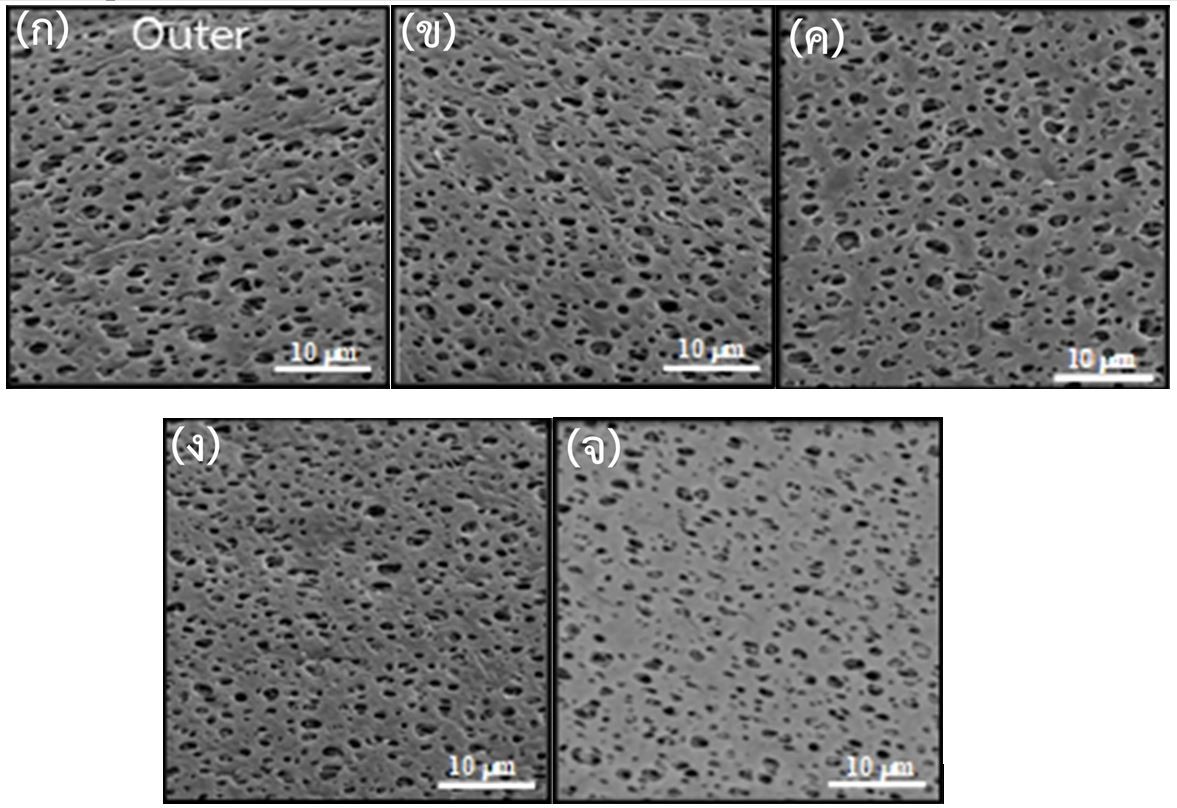
Results: This study showed no significant difference of the efficiency as urea reduction rate (URR), dialysis adequacy (Kt/V), and normalized protein catabolic rate (nPCR) between two groups of the patients using reused dialyzers. The result suggested that the PES membrane’s performance could be reused about 15 times. Visualization of the microstructure within the membranes was further investigated using a scanning electron microscope (SEM). The inner and outer surface areas of membranes upon the number of times for dialyzers reusable up to 10 and 15 times were corroded by peracetic acid as demonstrated by the membrane leakage and smaller membrane pore sizes, respectively. Additional evidence for the accumulation of peracetic acid leading to a destruction of the membrane structure was confirmed by energy dispersive X-ray spectroscopy (EDS). The EDS demonstrated high sulfur atoms in the inner surface area related with increased numbers of reused dialyzers. The interaction between the dialyzer membrane and peracetic acid activated many negatively charged ions absorption resulting in the membrane fouling.
Conclusion: The number of PES dialyzer reuses can be performed for 10 times since the structure of dialysis membrane do not change in their element compositions and structures with a peracetic acid sterilant.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
This article is published under CC BY-NC-ND 4.0 license, which allows for non-commercial reuse of the published paper as long as the published paper is fully attributed. Anyone can share (copy and redistribute) the material in any medium or format without having to ask permission from the author or the Nephrology Society of Thailand.
References
Said N, Lau WJ, Ho YC, Lim SK, Zainol Abidin MN, Ismail AF. A Review of Commercial Developments and Recent Laboratory Research of Dialyzers and Membranes for Hemodialysis Application. Membranes (Basel). 2021;11(10):767.
Wenten I.G APTP, Khoiruddin, Hakim A.N, Himma N.F. Advances in Polysulfone-Based Membranes for Hemodialysis. J Membr Sci Res. 2016;2:78-89.
Rohtash SA, Kumar R. Corrosion Study of Stainless Steels in Peracetic Acid Bleach Media With and Without Chloride and Chelant. IJRIST. 2014;1(1):1-10.
Kim J HC. Reactivity of Peracetic Acid with Organic Compounds: A Critical Review. ACS EST Water. 2021;1:15-23.
Shao J, Wolff S, Zydney AL. In vitro comparison of peracetic acid and bleach cleaning of polysulfone hemodialysis membranes. Artif Organs. 2007;31(6):452-60.
Matos JP, Andre MB, Rembold SM, Caldeira FE, Lugon JR. Effects of dialyzer reuse on the permeability of low-flux membranes. Am J Kidney Dis. 2000;35(5):839-44.
Gaudichet-Maurin E, Thominette F. Ageing of polysulfone ultrafiltration membranes in contact with bleach solutions. J Membrane Sci. 2006;282:198-204.
Tsehayeab MT WJ, Zhuac J, Velizarovd S, Bruggenae BV. Development and Characterization of Polyethersulfone-based Nanofiltration Membrane with Stability to Hydrogen Peroxide. J Membr Sci 2018;560:462-469.
Ward RA, Ouseph R. Impact of bleach cleaning on the performance of dialyzers with polysulfone membranes processed for reuse using peracetic Acid. Artif Organs. 2003; 27(11):1029-34.
Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002; 347(25):2010-9.
Rasband WS, ImageJ, U. S. National Institutes of Health. Bethesda, Maryland, USA, .
Mishra RK ZA, Thomas S. Energy-Dispersive X-ray Spectroscopy Techniques for Nanomaterial. Mahatma Gandhi University, Kottayam, Kerala, India; 2Mar Thoma College, Tiruvalla, Kerala, India: Elsevier Inc.; 2017.
Sun Y XL, Zhang Y, Zhao X, Huang Y, Du X. High flux polyamide thin film composite forward osmosis membranes prepared from porous substrates made of polysulfone and polyethersulfone blends. Desalination. 2014;336:72-9.
Mohammad AW TY, Chong WC, Ho KC. Hybrid Processes: Membrane Bioreactor. Centre for Sustainable Process Technology (CESPRO), Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, Selangor, Malaysia.2019.
Olesberg JT, Arnold MA, Flanigan MJ. Online measurement of urea concentration in spent dialysate during hemodialysis. Clin Chem. 2004;50(1):175-81.
Goodman BE. Transport of small molecules across cell membranes: water channels and urea transporters. Adv Physiol Educ. 2002;26:146-57.
Syawaliah MS, Muzaitun, Mulyasari R. Characterization of Polydopamine-Coated Polyethersulfone (PES) membrane for water purification. The 7th AIC-ICMR on Sciences and Engineering 2017: IOP Publishing; 2018. p. 1-7.
Olawumi O Sadare O, Daramola. Blended Polysulfone/Polyethersulfone (PSF/PES) Membrane with Enhanced Antifouling Property for Separation of Succinate from Organic Acids from Fermentation Broth. ACS Sustainable Chem Eng. 2021;9:13068-83.
Chipiso K, Logan IE, Eskew MW, Omondi B, Simoyi RH. Kinetics and Mechanism of Bioactivation via S-Oxygenation of Anti-Tubercular Agent Ethionamide by Peracetic Acid. J Phys Chem A. 2016;120(41):8056-64.
Waninksamban W LB, Tongdee C, Spilles N, Ussawawongaraya W. The peracetic acid rebound after rinsing procedure in dialyzer reuse. Journal of the nephrology society of Thailand. 2016;2:50-4.
Townsend DM, Tew KD, Tapiero H. Sulfur containing amino acids and human disease. Biomed Pharmacother. 2004;58(1):47-55.
Sahebi S, Kahrizi M, Fadaie N, Hadadpour S, Ramavandi B, Gonzales RR. Developing a Thin Film Composite Membrane with Hydrophilic Sulfonated Substrate on Nonwoven Backing Fabric Support for Forward Osmosis. Membranes (Basel). 2021;11(11):813.
Gasch J LC, Knoth H. Positively Charged Polyethersulfone Membranes: The Influence of Furosemide on the Zeta Potential. J Membra Sci Technol. 2013;3(1):1-5.
Alenazi NA, Hussein MA, Alamry KA, Asiri AM. Modified polyether-sulfone membrane: a mini review. Des Monomers Polym. 2017;20(1):532-46.