Editorial Policy
Editorial Policy
Focus and Scope
Chulalongkorn Medical Journal aims to publish original peer-reviewed contributions related with various aspects in the biomedical and health sciences from experimental to clinical topics. Original research articles, short communications, review articles, case reports, and letter to editor are welcome.
The journal is supported by the Faculty of Medicine, Chulalongkorn University, who covers the cost of publication on behalf of the authors on acceptance of their article. Contemporary studies including basic research, clinical trials, studies of diagnostic accuracy, studies relating to behavioral, therapeutic, or epidemiological aspects of medicine, public health, environmental health, and health and social care are welcomed. We also welcome research from disciplines across the health sciences.
Section Policies
Peer Review Process
Peer review is the system used to assess the quality of a manuscript before it is published. Independent researchers in the relevant research area assess submitted manuscripts for originality, validity and significance to help editors determine whether the manuscript should be published in their journal. More details about the peer review process can be found here.
Chulalongkorn Medical Journal operates a double-blind peer review system, where both the reviewer and author identities are concealed from the reviewers, and vice versa, throughout the review process. It is the traditional model of peer review that many reviewers are comfortable with, and it facilitates an unbiased assessment of a manuscript.
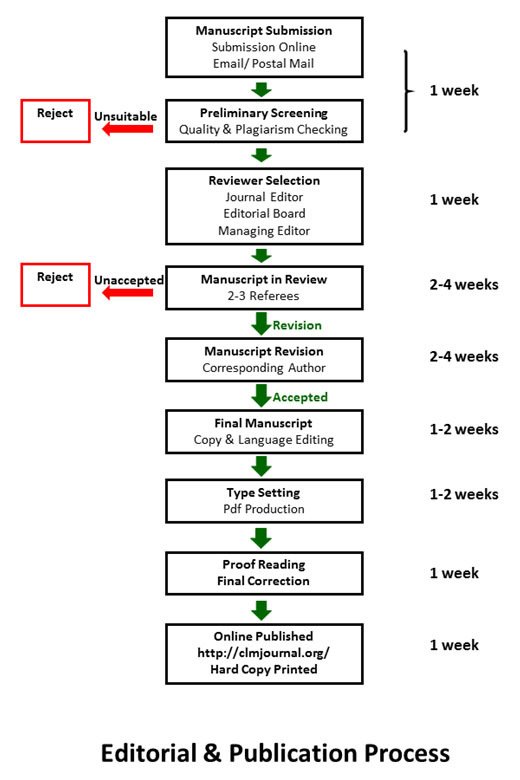
Chulalongkorn Medical Journal is supported by an expert Editorial Board. All submitted manuscripts are evaluated by the Editor-in-Chief and appropriate manuscripts are then assigned to at least two reviewers for their comments. We make every effort to reach an initial decision within six weeks of submission. Based on the reviews, the Editor-in-Chief rejects, accepts or requests revisions of the manuscrip
Publication Frequency
All manuscripts submitted to the Chulalongkorn Medical Journal are first evaluated on the basis of scientific quality, originality, appropriateness, contribution to the field, and style. Suitable manuscripts are then subject to rigorous, fair, and rapid peer review.
Issues per year: Quarterly (10 - 15 articles per issue)
No.1 January - March
No.2 April - June
No.3 July - September
No.4 October - December
Open Access Policy
All articles published by the Chulalongkorn Medical Journal are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers. As authors of articles published in the Chulalongkorn Medical Journal, they are the copyright holders of their own article and have granted to any third party, in advance and in perpetuity, the right to use, reproduce or disseminate their article. Our authors retain copyright of their work through a Creative Commons attribution license that clearly states how readers can copy, distribute, and use their attributed research, free of charge.
Conflict of Interest
The corresponding author of an article is asked to inform the Editor of the authors' potential conflicts of interest possibly influencing the research or interpretation of data. A potential conflict of interest should be disclosed in the cover letter even when the authors are confident that their judgments have not been influenced in preparing the manuscript. Such conflicts may include financial support or private connections to pharmaceutical companies, political pressure from interest groups, or academic problems. Disclosure form shall be same with the International Committee of Medical Journal Editors (ICMJE) Uniform Disclosure Form for Potential Conflicts of Interest (http://www.icmje.org/coi_disclosure.pdf). The Editor will decide whether the information on the conflict should be included in the published paper. In particular, all sources of funding for a study should be explicitly stated. The Chulalongkorn Medical Journal asks referees to let its Editor know of any conflict of interest before reviewing a particular manuscript.
Publication Ethics
Authorship
All the listed authors must have agreed all of the contents, including the author list and author contributions statements. The corresponding author is responsible for having ensured that this agreement has been reached, that all authors have agreed to be listed and approved the manuscript submission to the journal, and for managing all communication between the journal and all co-authors, before and after publication. Any changes to the author list after submission needs to be approved by every author.
Originality and plagiarism
Manuscripts submitted to the Chulalongkorn Medical Journal must not been published previously and is not under consideration for publication elsewhere. Authors should adhere to publication requirements that submitted work is original, is not plagiarized, and has not been published elsewhere. Fraudulent or knowingly inaccurate statements constitute unethical behavior and are unacceptable. Authors should not in general publish manuscripts describing essentially the same research in more than one journal or primary publication. Submitting the same manuscript to more than one journal concurrently constitutes unethical publishing behavior and is unacceptable.
Human or animal subjects
If the work involves the use of animal or human subjects, the author should ensure that the manuscript constitutes statements of compliance with relevant institutional guidelines. Studies on human and animals require the approval from the institutional review board. Authors should provide a statement in the manuscript that informed consent was acquired for experimentation with human subjects.
Authors should include a statement in the manuscript that informed consent was obtained for experimentation with human subjects. The privacy rights of human subjects must always be observed. For investigations involving human participants or data, or human material samples, or animal studies or samples, appropriate institutional review board or ethics committee approval is required, and such approval must be stated in the Methods section of the manuscript. Research involving human subjects, identifiable human samples (such as urine, blood, serum, or tissue), and personal and health record data must be subjected to a review by a formally constituted institutional ethical review board. Studies must in any case be in accordance with the principles outlined in the contemporary revision of the Declaration of Helsinki of 1964 (World Medical Association (WMA) incorporating the most recent (October 2013) and earlier amendments.
Procedures involving any animal are to be undertaken only with the goal of advancing scientific knowledge and with the explicit approval of an Institutional Animal Care and Use Committee (IACUC) before they begin. All animal experiments must conform to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines or the revised Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council “Guide for the Care and Use of Laboratory Animals” Washington, D.C.: National Academy Press; 1996. The European Commission Directive 2010/63/EU revising Directive 86/609/ EEC for animal experiments, the revised Animals (Scientific Procedures) Act (ASPA) 1986 in the UK, or The Animals for Scientific Purposes Act, BE 2558 (AD 2015), which regulates the use of both vertebrates and invertebrates without exception and must be followed for all studies conducted in Thailand. The International Association of Veterinary Editors’ Consensus Author Guidelines on Animal Ethics and Welfare provide further guidance. The authors should clearly indicate in the manuscript that such guidelines have been followed. The sex of animals must be indicated, and where appropriate, the influence (or association) of sex on the results of the study.
Informed Consent
All authors are required to follow the International Committee of Medical Journal Editors (ICMJE) requirements on privacy and informed consent from patients and study participants. Please confirm that any patient, service user, or participant (or that person's parent or legal guardian) in any research, experiment, or clinical trial described in the authors’ paper has given written consent to the inclusion of material with regard to themselves, that they acknowledge that they cannot be identified via the paper; and that the authors have fully anonymized them. Where someone is deceased, please ensure the authors have written consent from the family or estate.
Editorial ethics
All of the manuscripts should be prepared based on strict observation of research and publication ethics guidelines recommended by the Council of Science Editors (http://www.councilscienceeditors.org/), the International Committee of Medical Journal Editors (ICMJE, http://www.icmje.org/), and the World Association of Medical Editors (WAME, http://www.wame.org/). All studies involving human subjects or human data must be reviewed and approved by a responsible Institutional Review Board (IRB). Please refer to the principles embodied in the Declaration of Helsinki (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/) for all investigations involving human materials. Animal experiments also should be reviewed by an appropriate committee (IACUC) for the care and use of animals. Also studies with pathogens requiring a high degree of biosafety should pass review of a relevant committee (IBC). The approval should be described in the Methods section. For studies of humans including case reports, state whether informed consents were obtained from the study participants. The editor of Chulalongkorn Medical Journal may request submission of copies of informed consents from human subjects in clinical studies or IRB approval documents. The Chulalongkorn Medical Journal follows the guidelines by the Committee on Publication Ethics (COPE, http://publicationethics.org/) that set standards and provide guidelines for best practices in order to meet these requirements. The journal adheres to ensure the standards of expected ethical behavior for all parties related to the act of authors, peer reviewers, and editors.
Duties of Authors
- Authors should provide an accurate account of the work performed as well as an objective discussion of its significance.
- The authors should ensure their original works, and if the authors have applied the work and/or words of others, that this has been appropriately quoted and permission has been acquired where necessary.
- The authors should present their findings clearly, honestly without fabrication, falsification or inappropriate manner.
- The authors describe their methods unambiguously, therefore their results can be reproducible.
- Authors should not publish manuscripts describing essentially the same research in more than one journal of primary publication. Multiple, redundant or concurrent Publication is unacceptable.
- Authorship should reflect individuals who have made a significant contribution to the conception, design, execution, or interpretation of the reported study. All those who have made substantial contributions should be listed as co-authors.
- All sources of funding and potential conflicts of interest should be disclosed.
- The corresponding author must confirm that the corresponding author had final responsibility for the decision to submit for publication and had full access to all the data in the study.
Duties of Reviewers
- The reviewers should provide prompt, timely, and unbiased response to the Editors on manuscripts.
- The reviewers should alert the editors of any possible reasons or potential conflicts of interest involved in a manuscript or its authors, or knowledge of a study, and withdraw from the process.
- The reviewers should keep confidentiality of the entire documents in the review process.
- The reviewers should notify the editors of any plagiarism where authors have not quoted or cited appropriately or duplicated work in publications, including their own.
- The reviewers should provide timely feedback to authors who do not personally criticize them and provides clear supportive statements to back up their review decisions.
- The reviewers should notify the editor and decline to participate in the review process if they feel unqualified or do not have the expertise to review the research reported in a manuscript.
- The reviewers never use unpublished materials disclosed in the review process in their own research without permission of the authors.
Duties of Editors
- The editor is solely and independently responsible for deciding which of the articles should be published. The editor may consult editorial board or reviewers in making the decisions.
- The editor should ensure that the peer review process is fair, unbiased, and timely.
- The editor should select reviewers who have suitable expertise in the relevant field and shall follow best practice in avoiding the selection of fraudulent peer reviewers
- Editors should guard the integrity of the published record by issuing corrections and retractions when needed and pursuing suspected or alleged research and publication misconduct.
- Editors should critically assess the ethical conduct of studies in humans and animals.
- Editors should have appropriate policies in place for handling editorial conflicts of interest.
- The editor must protect the confidentiality of all material submitted to the journal.
Publication Process
Copyright & Licensing
The journal adopts CC BY-NC-ND license for its content. Users are free to:
- Share: Copy and redistribute the material in any medium or format.
- Attribution: You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
- Non Commercial: You may not use the material for commercial purposes.
- No Derivatives: If you remix, transform, or build upon the material, you may not distribute the modified material.
- No additional restrictions: You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits.
- The non-commercial use of the article will be governed by the Creative Commons Attribution-NonCommercial-NoDerivs license as currently displayed on http://creativecommons.org/licenses/by-nc-nd/3.0/, except that sections 2 through 8 below will apply in this respect and prevail over all conflicting provisions of such license model. Without prejudice to the foregoing, the author hereby grants the Journal Owner the exclusive license for commercial use of the article (for U.S. government employees: to the extent transferable) according to section 2 below, and sections 4 through 9 below, throughout the world, in any form, in any language, for the full term of copyright, effective upon acceptance for publication.
- Under the Creative Commons Attribution-NonCommercial-NoDerivs license, the author(s) and users are free to share (copy, distribute and transmit the contribution) under the following conditions: 1. they must attribute the contribution in the manner specified by the author or licensor, 2. they may not use this contribution for commercial purposes, 3. they may not alter, transform, or build upon this work.